Ionisation of water and p H concept p
![Ionisation of water and p. H concept p. H = -log[H+] p. X = Ionisation of water and p. H concept p. H = -log[H+] p. X =](https://slidetodoc.com/presentation_image/32bfcc3f0c3805ab26a058272ff193a8/image-1.jpg)

- Slides: 13
![Ionisation of water and p H concept p H logH p X Ionisation of water and p. H concept p. H = -log[H+] p. X =](https://slidetodoc.com/presentation_image/32bfcc3f0c3805ab26a058272ff193a8/image-1.jpg)
Ionisation of water and p. H concept p. H = -log[H+] p. X = -log. X p. H scale [H+] > 10 -7 M, p. H < 7 ACIDIC [H+] < 10 -7 M, p. H > 7 For any Bronsted conjugate Acid-Base pair Ka. Kb = Kw BASIC [H+] = 10 -7 M, p. H = 7 NEUTRAL

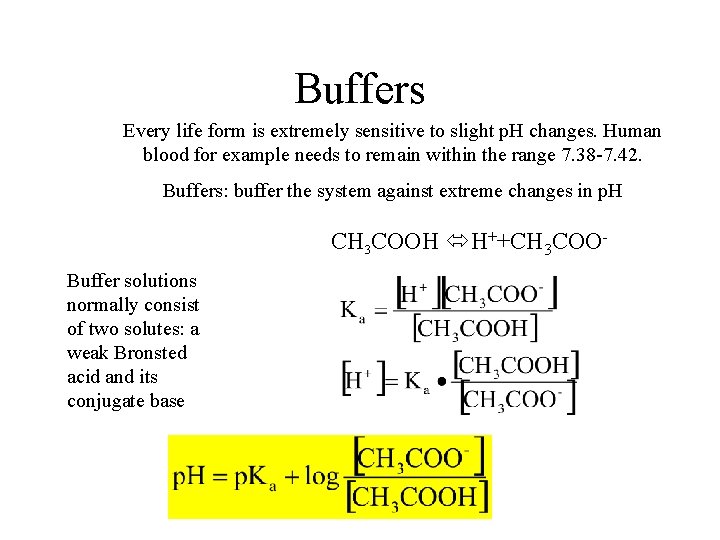
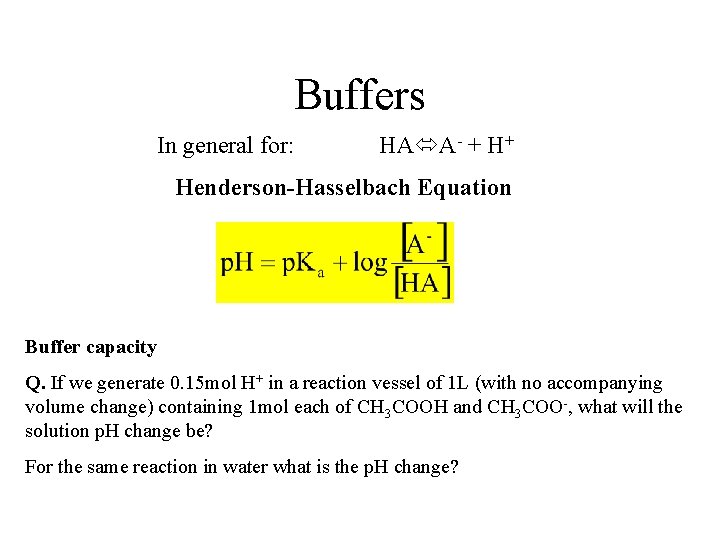
Buffers Every life form is extremely sensitive to slight p. H changes. Human blood for example needs to remain within the range 7. 38 -7. 42. Buffers: buffer the system against extreme changes in p. H CH 3 COOH H++CH 3 COOBuffer solutions normally consist of two solutes: a weak Bronsted acid and its conjugate base

Buffers In general for: HA A- + H+ Henderson-Hasselbach Equation Buffer capacity Q. If we generate 0. 15 mol H+ in a reaction vessel of 1 L (with no accompanying volume change) containing 1 mol each of CH 3 COOH and CH 3 COO-, what will the solution p. H change be? For the same reaction in water what is the p. H change?

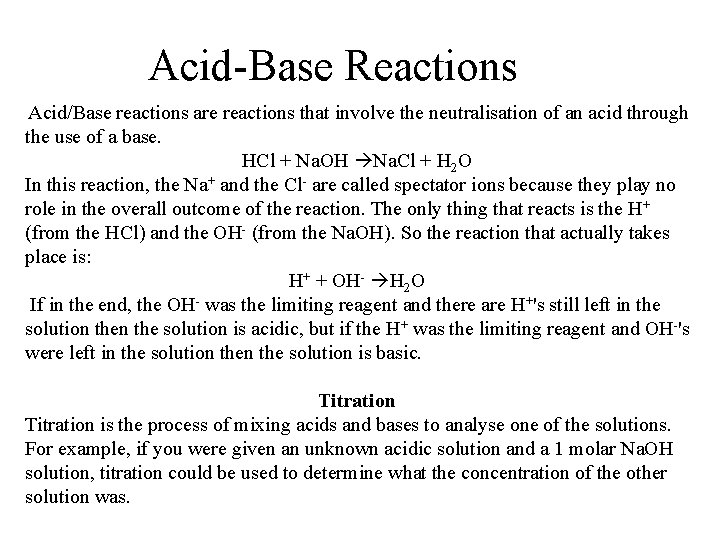
Acid-Base Reactions Acid/Base reactions are reactions that involve the neutralisation of an acid through the use of a base. HCl + Na. OH Na. Cl + H 2 O In this reaction, the Na+ and the Cl- are called spectator ions because they play no role in the overall outcome of the reaction. The only thing that reacts is the H+ (from the HCl) and the OH- (from the Na. OH). So the reaction that actually takes place is: H+ + OH- H 2 O If in the end, the OH- was the limiting reagent and there are H+'s still left in the solution is acidic, but if the H+ was the limiting reagent and OH-'s were left in the solution is basic. Titration is the process of mixing acids and bases to analyse one of the solutions. For example, if you were given an unknown acidic solution and a 1 molar Na. OH solution, titration could be used to determine what the concentration of the other solution was.

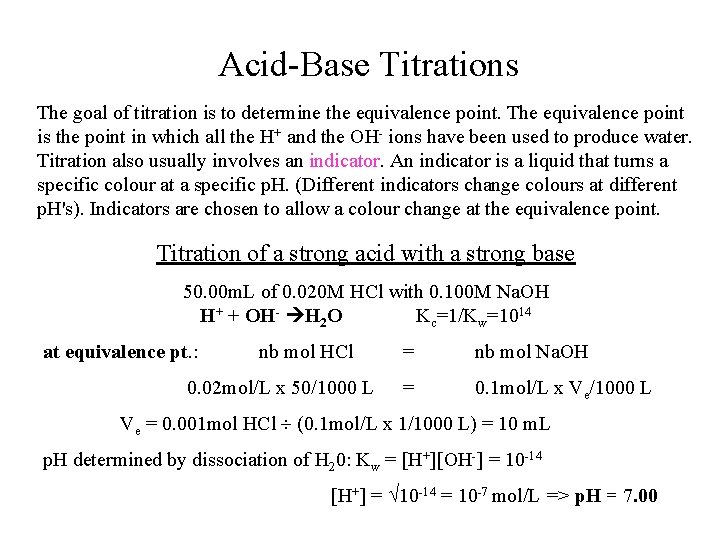
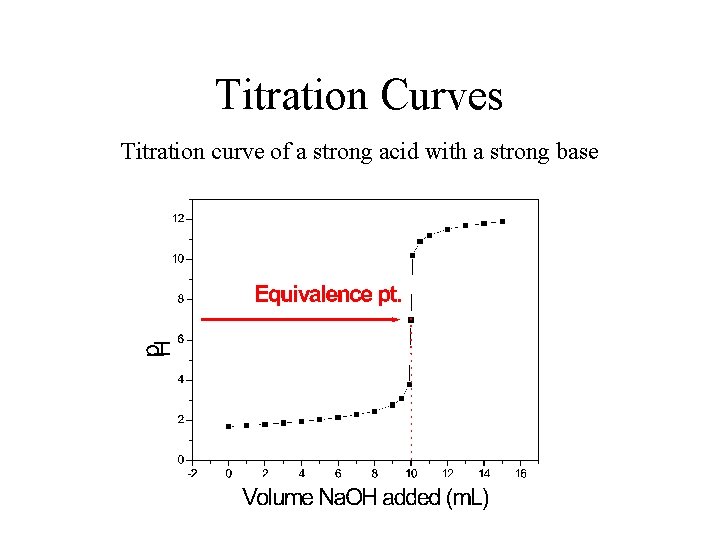
Acid-Base Titrations The goal of titration is to determine the equivalence point. The equivalence point is the point in which all the H+ and the OH- ions have been used to produce water. Titration also usually involves an indicator. An indicator is a liquid that turns a specific colour at a specific p. H. (Different indicators change colours at different p. H's). Indicators are chosen to allow a colour change at the equivalence point. Titration of a strong acid with a strong base 50. 00 m. L of 0. 020 M HCl with 0. 100 M Na. OH H+ + OH- H 2 O Kc=1/Kw=1014 at equivalence pt. : nb mol HCl 0. 02 mol/L x 50/1000 L = nb mol Na. OH = 0. 1 mol/L x Ve/1000 L Ve = 0. 001 mol HCl (0. 1 mol/L x 1/1000 L) = 10 m. L p. H determined by dissociation of H 20: Kw = [H+][OH-] = 10 -14 [H+] = 10 -14 = 10 -7 mol/L => p. H = 7. 00

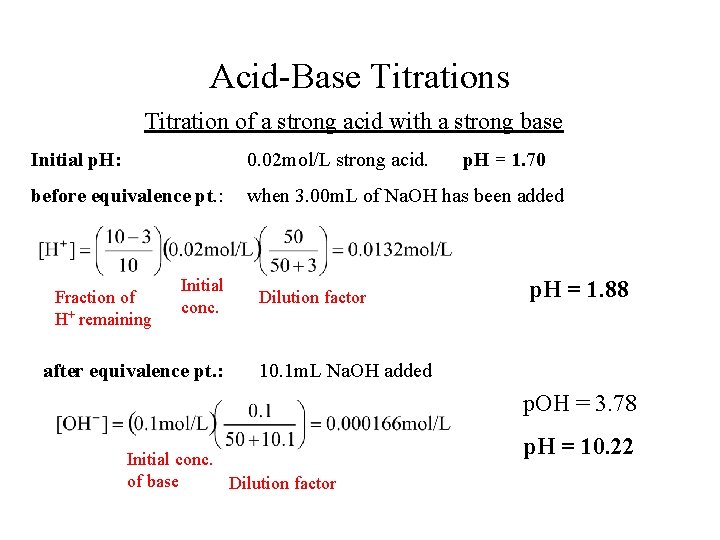
Acid-Base Titrations Titration of a strong acid with a strong base Initial p. H: 0. 02 mol/L strong acid. p. H = 1. 70 before equivalence pt. : when 3. 00 m. L of Na. OH has been added Fraction of H+ remaining Initial conc. after equivalence pt. : Dilution factor p. H = 1. 88 10. 1 m. L Na. OH added p. OH = 3. 78 Initial conc. of base Dilution factor p. H = 10. 22

Titration Curves Titration curve of a strong acid with a strong base

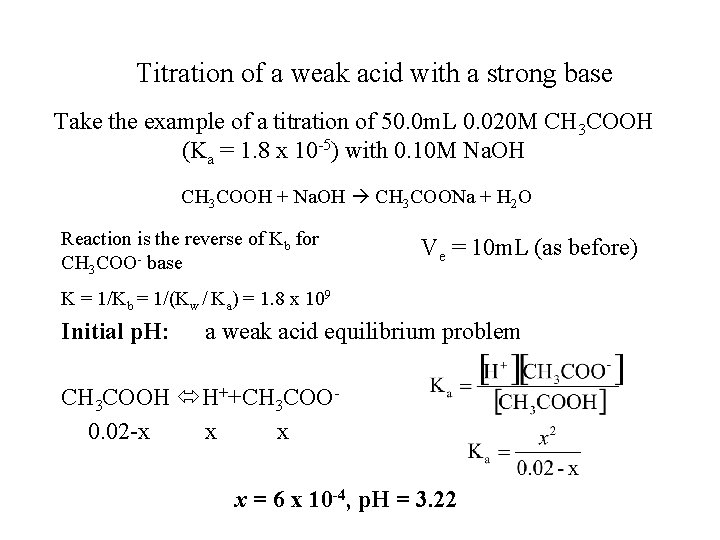
Titration of a weak acid with a strong base Take the example of a titration of 50. 0 m. L 0. 020 M CH 3 COOH (Ka = 1. 8 x 10 -5) with 0. 10 M Na. OH CH 3 COOH + Na. OH CH 3 COONa + H 2 O Reaction is the reverse of Kb for CH 3 COO- base Ve = 10 m. L (as before) K = 1/Kb = 1/(Kw / Ka) = 1. 8 x 109 Initial p. H: a weak acid equilibrium problem CH 3 COOH H++CH 3 COO 0. 02 -x x = 6 x 10 -4, p. H = 3. 22

Titration of a weak acid with a strong base Before eq. pt. : buffer system One of the simplest ways to treat these problems is to evaluate the quotient in the log using relative concentration before and after the reaction. Imagine we have added 3. 00 m. Ls of base CH 3 COOH + Na. OH CH 3 COONa + H 2 O Relative Initial: 1 Relative final: 7/10 3/10

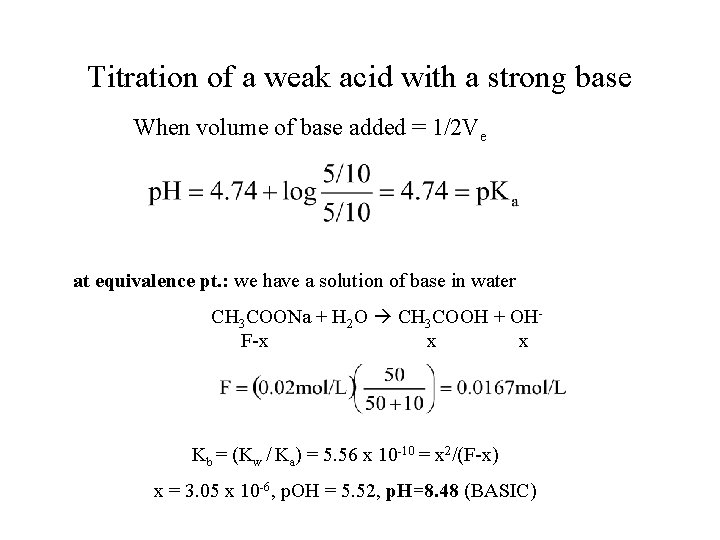
Titration of a weak acid with a strong base When volume of base added = 1/2 Ve at equivalence pt. : we have a solution of base in water CH 3 COONa + H 2 O CH 3 COOH + OH F-x x x Kb = (Kw / Ka) = 5. 56 x 10 -10 = x 2/(F-x) x = 3. 05 x 10 -6, p. OH = 5. 52, p. H=8. 48 (BASIC)

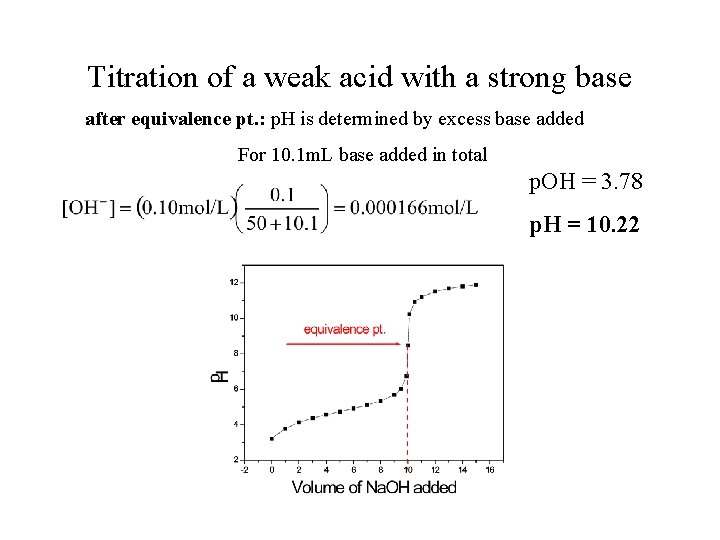
Titration of a weak acid with a strong base after equivalence pt. : p. H is determined by excess base added For 10. 1 m. L base added in total p. OH = 3. 78 p. H = 10. 22

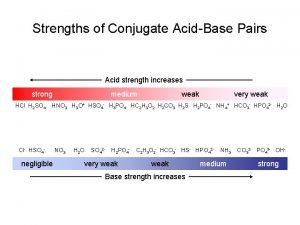
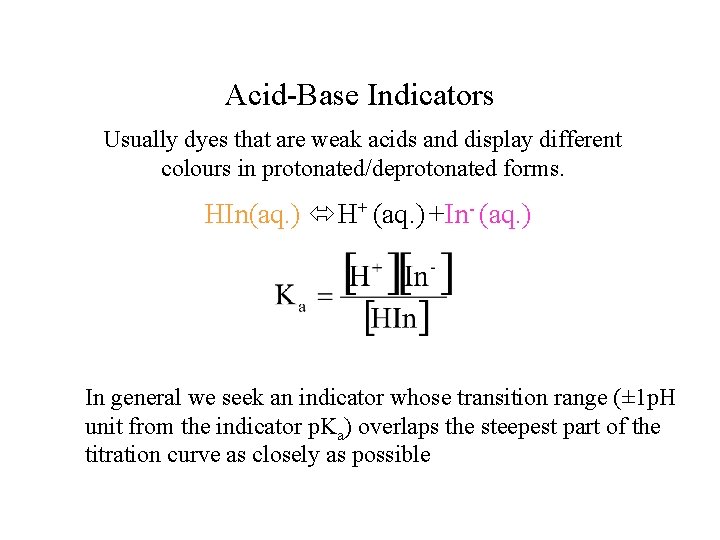
Acid-Base Indicators Usually dyes that are weak acids and display different colours in protonated/deprotonated forms. HIn(aq. ) H+ (aq. ) +In- (aq. ) In general we seek an indicator whose transition range (± 1 p. H unit from the indicator p. Ka) overlaps the steepest part of the titration curve as closely as possible

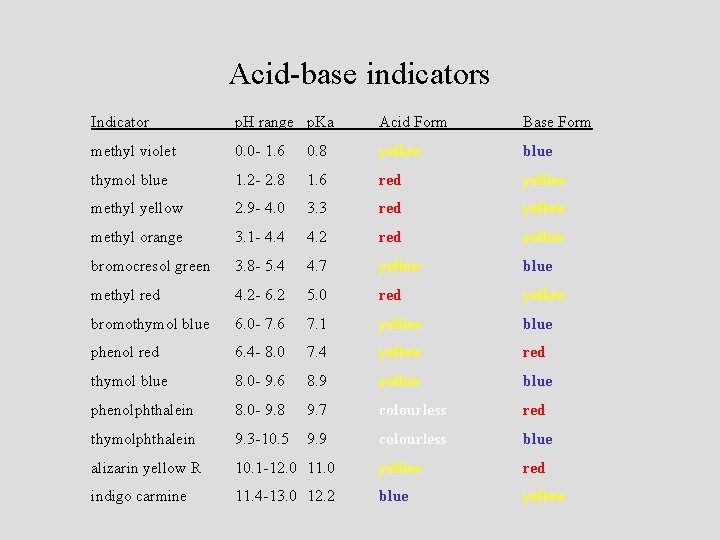
Acid-base indicators Indicator p. H range p. Ka Acid Form Base Form methyl violet 0. 0 - 1. 6 0. 8 yellow blue thymol blue 1. 2 - 2. 8 1. 6 red yellow methyl yellow 2. 9 - 4. 0 3. 3 red yellow methyl orange 3. 1 - 4. 4 4. 2 red yellow bromocresol green 3. 8 - 5. 4 yellow blue methyl red 4. 2 - 6. 2 5. 0 red yellow bromothymol blue 6. 0 - 7. 6 7. 1 yellow blue phenol red 6. 4 - 8. 0 7. 4 yellow red thymol blue 8. 0 - 9. 6 8. 9 yellow blue phenolphthalein 8. 0 - 9. 8 9. 7 colourless red thymolphthalein 9. 3 -10. 5 9. 9 colourless blue alizarin yellow R 10. 1 -12. 0 11. 0 yellow red indigo carmine 11. 4 -13. 0 12. 2 blue yellow 4. 7
 Water and water and water water
Water and water and water water Ph 3 acid or base
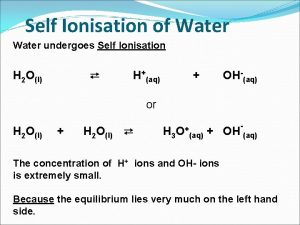
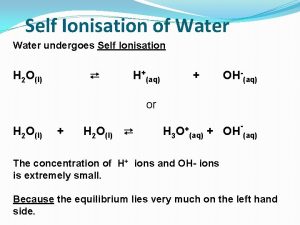
Ph 3 acid or base Self ionisation of water
Self ionisation of water Self ionization of water
Self ionization of water Ionization of water
Ionization of water Electronegativity and atom size
Electronegativity and atom size Ib chemistry atomic structure
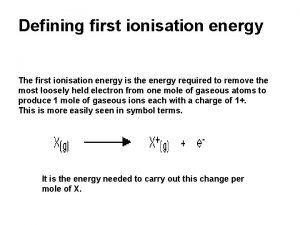
Ib chemistry atomic structure Definition first ionisation energy
Definition first ionisation energy Acid and base properties
Acid and base properties Hydrochloric acid ionization equation
Hydrochloric acid ionization equation Trend going down
Trend going down Successive ionisation energies of calcium
Successive ionisation energies of calcium Ionisation varèse analisi
Ionisation varèse analisi Ionisation meaning
Ionisation meaning