Introduction Most of the nanoparticles only emit a



- Slides: 12


Introduction Most of the nanoparticles only emit a single fluorescence Can be interfered by autofluorescence or background noise complexity Solution: photoswitchable fluorescent polymeric nanoparticles (PFPNs) Based on the fluorescence resonance energy transfer (FRET) strategy In general, PFPNs contains photochromic component as an energy acceptor and a fluorescence component as an energy donor In this case, energy acceptor is spiropyran (SP) and energy donor is naphtalimide (NAPH) Photonsensitive groups in the nanoparticles can convert photoirradiation to chemical signal through photoreactions Photoisomerization That can lead to the controlled release of encapsulated molecules in the nanoparticles

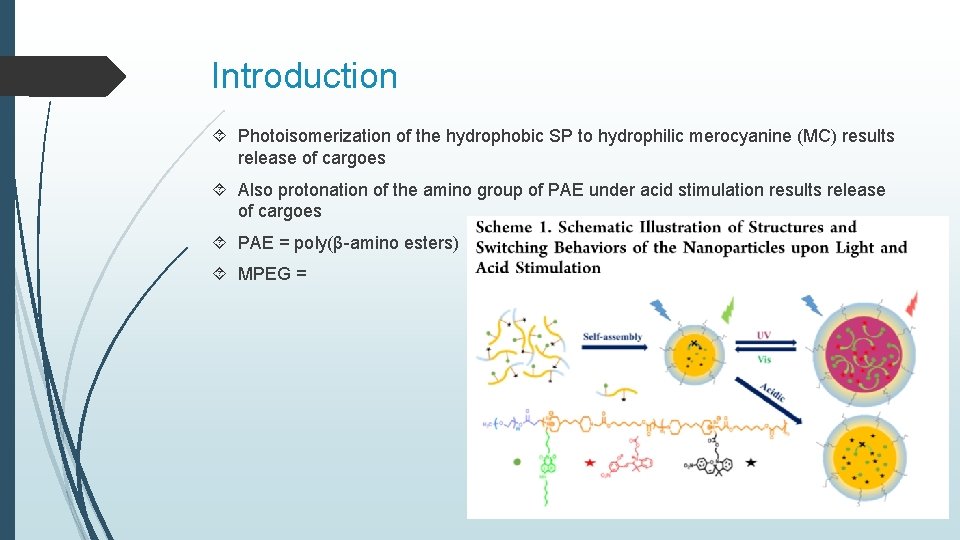
Introduction Photoisomerization of the hydrophobic SP to hydrophilic merocyanine (MC) results release of cargoes Also protonation of the amino group of PAE under acid stimulation results release of cargoes PAE = poly(β-amino esters) MPEG =

Experimental methods Synthesizing spiropyran (SP) derivative (SP-Br) Synthesizing naphtalamide (NAPH) derivative (NAPH-Br) Synthesizing MPEG-PAE with Michael-type step Synthesizing copolymers containing SP and NAPH via quaternization Characterization H 1 -NMR Functional groups with FTIR Average molecular weight and molecular weight distribution with GPC The isomerization between SP and MC was switched using a UV LED lamp and a visible LED lamp

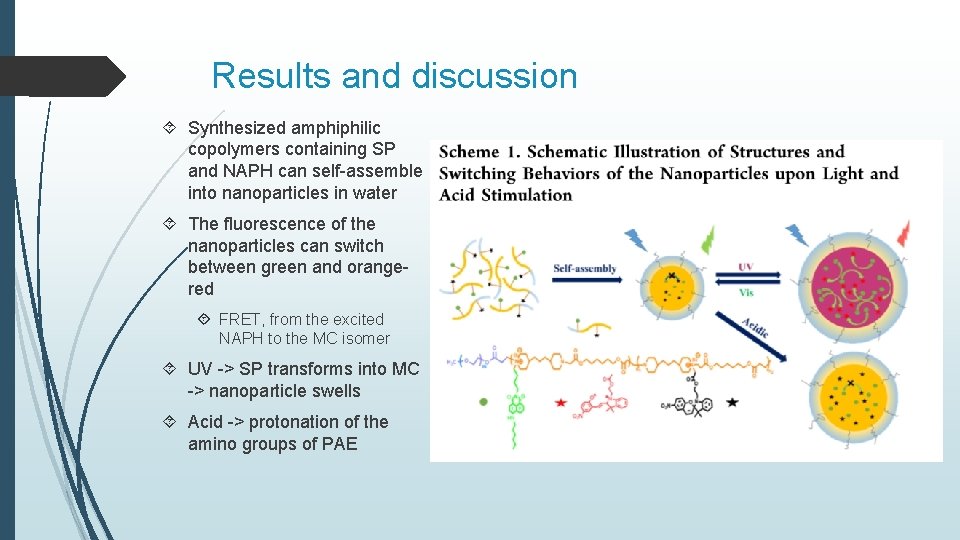
Results and discussion Synthesized amphiphilic copolymers containing SP and NAPH can self-assemble into nanoparticles in water The fluorescence of the nanoparticles can switch between green and orangered FRET, from the excited NAPH to the MC isomer UV -> SP transforms into MC -> nanoparticle swells Acid -> protonation of the amino groups of PAE

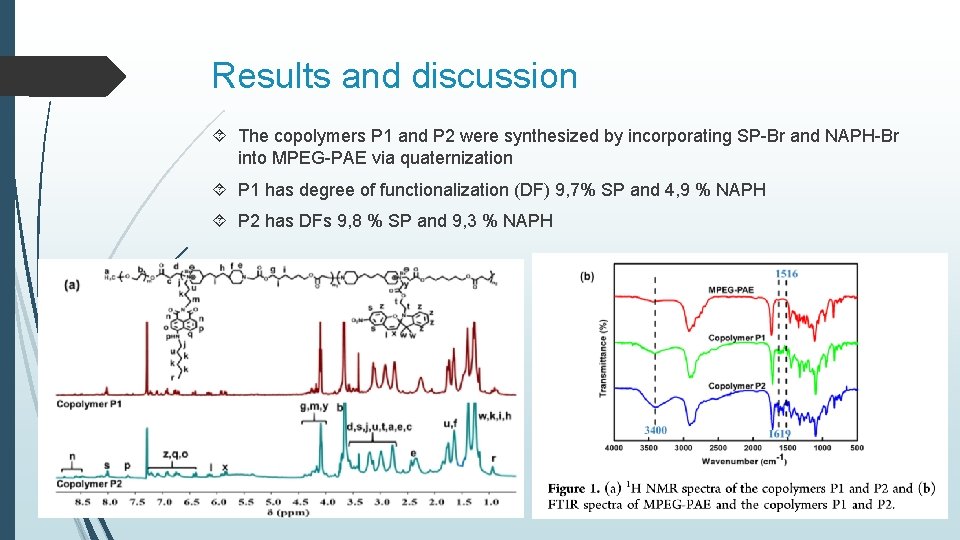
Results and discussion The copolymers P 1 and P 2 were synthesized by incorporating SP-Br and NAPH-Br into MPEG-PAE via quaternization P 1 has degree of functionalization (DF) 9, 7% SP and 4, 9 % NAPH P 2 has DFs 9, 8 % SP and 9, 3 % NAPH

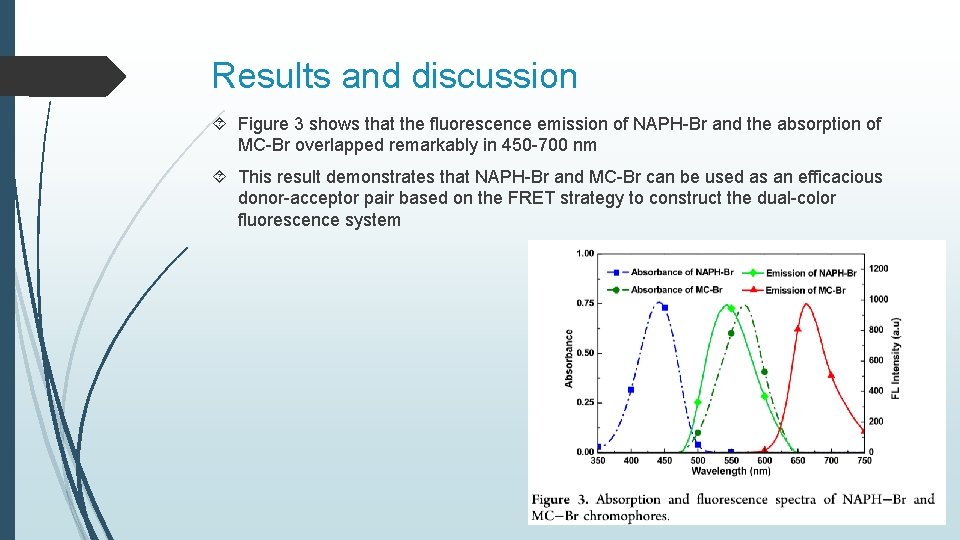
Results and discussion Figure 3 shows that the fluorescence emission of NAPH-Br and the absorption of MC-Br overlapped remarkably in 450 -700 nm This result demonstrates that NAPH-Br and MC-Br can be used as an efficacious donor-acceptor pair based on the FRET strategy to construct the dual-color fluorescence system

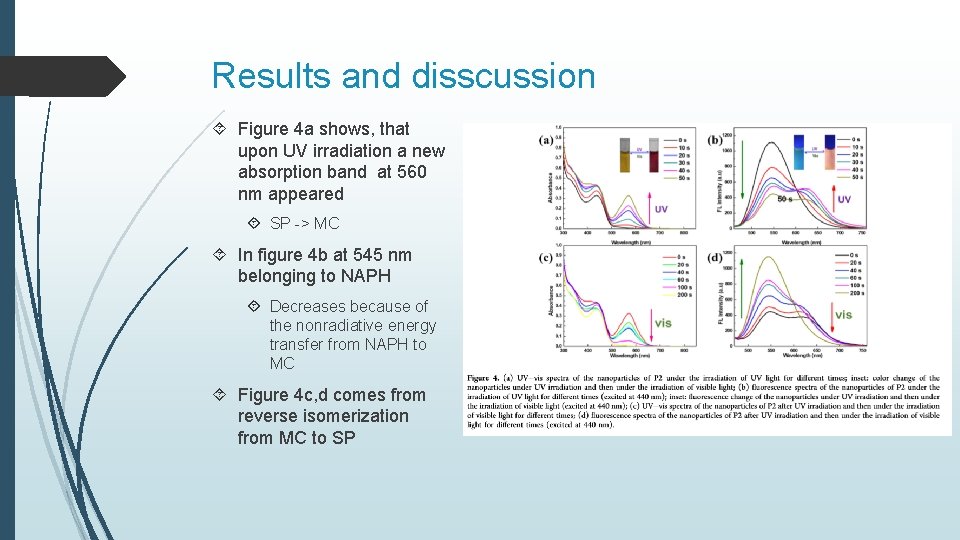
Results and disscussion Figure 4 a shows, that upon UV irradiation a new absorption band at 560 nm appeared SP -> MC In figure 4 b at 545 nm belonging to NAPH Decreases because of the nonradiative energy transfer from NAPH to MC Figure 4 c, d comes from reverse isomerization from MC to SP

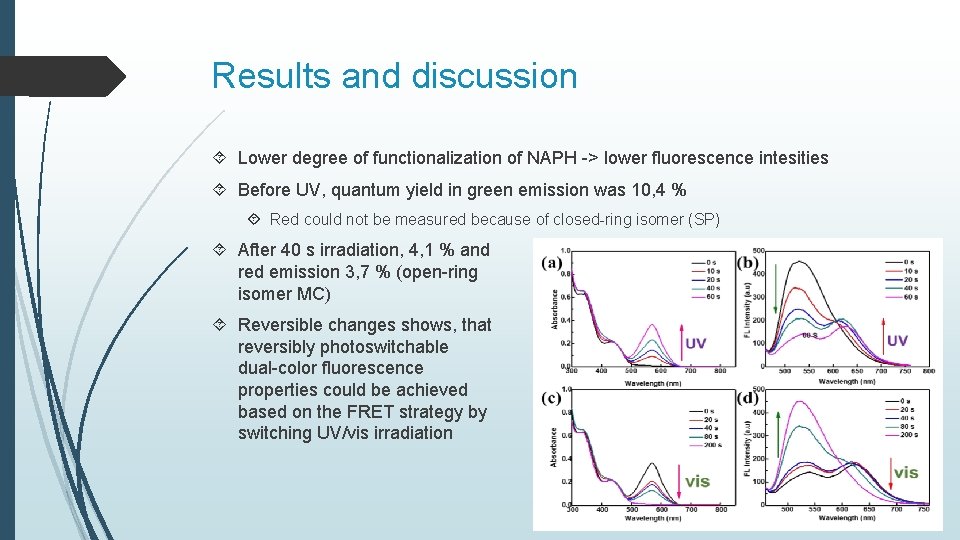
Results and discussion Lower degree of functionalization of NAPH -> lower fluorescence intesities Before UV, quantum yield in green emission was 10, 4 % Red could not be measured because of closed-ring isomer (SP) After 40 s irradiation, 4, 1 % and red emission 3, 7 % (open-ring isomer MC) Reversible changes shows, that reversibly photoswitchable dual-color fluorescence properties could be achieved based on the FRET strategy by switching UV/vis irradiation

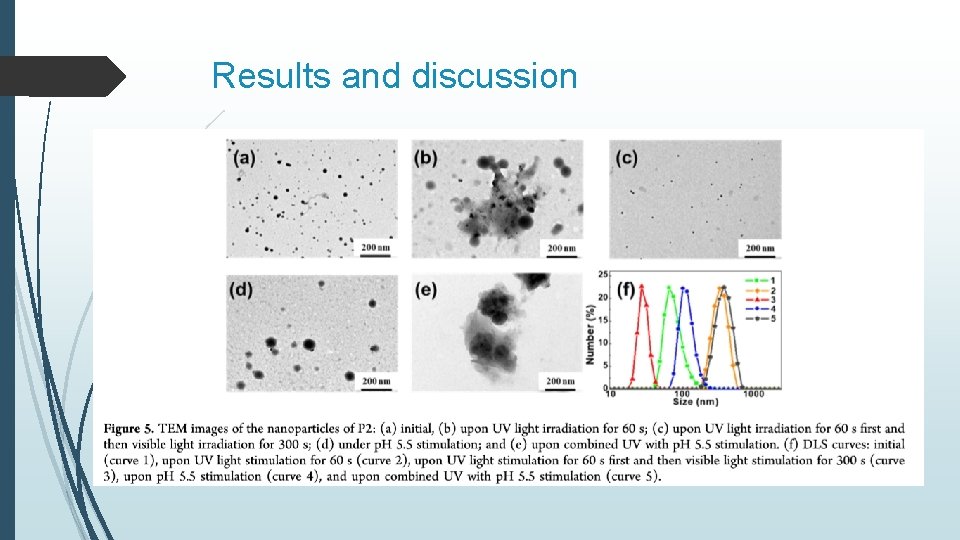
Results and discussion

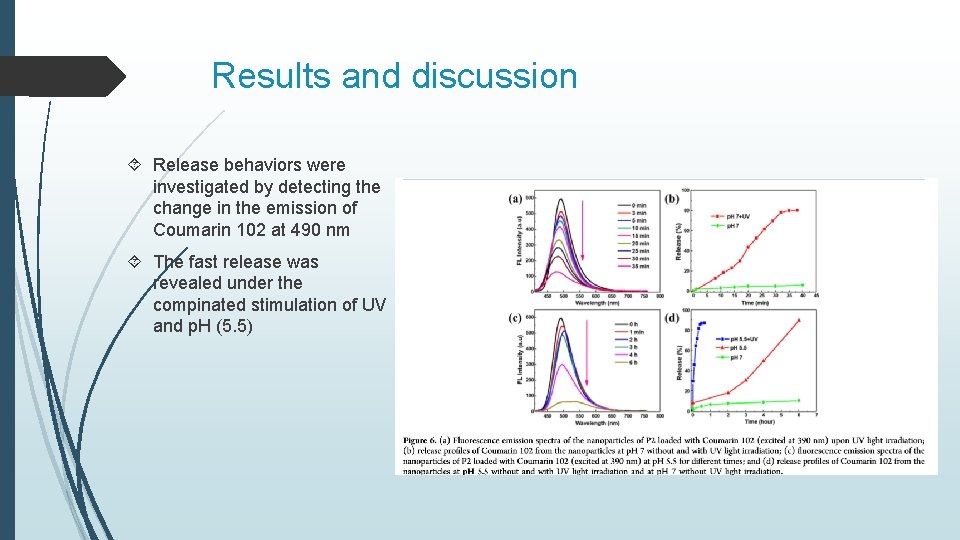
Results and discussion Release behaviors were investigated by detecting the change in the emission of Coumarin 102 at 490 nm The fast release was revealed under the compinated stimulation of UV and p. H (5. 5)

Conclusions Successful synthesis of a novel copolymer (NAPH, SP, MPEG-PAE) Morphological changes of the nanoparticles under the stimuli of UV light and acid Hydrophobic cargoes could be encapsulated through hydrophobic interaction and then released in response to UV light and acid





















