Institute of Organic Chemistry National Academy of Sciences

![2, 6 -DISUBSTITUTED SPIRO[3. 3]HEPTANES Chernykh, A. V. ; Volochnyuk D. M. Journal of 2, 6 -DISUBSTITUTED SPIRO[3. 3]HEPTANES Chernykh, A. V. ; Volochnyuk D. M. Journal of](https://slidetodoc.com/presentation_image_h2/5f97f5cf1feb81e59ccd4a14bcfc607a/image-12.jpg)
![New heterocyclic system of pyrrolo[3, 4 -d][1, 2]diazepine pyrrole analogues of antagonists of AMPA-receptor New heterocyclic system of pyrrolo[3, 4 -d][1, 2]diazepine pyrrole analogues of antagonists of AMPA-receptor](https://slidetodoc.com/presentation_image_h2/5f97f5cf1feb81e59ccd4a14bcfc607a/image-13.jpg)
- Slides: 14

Institute of Organic Chemistry National Academy of Sciences of Ukraine Scientific Report on 2015 year

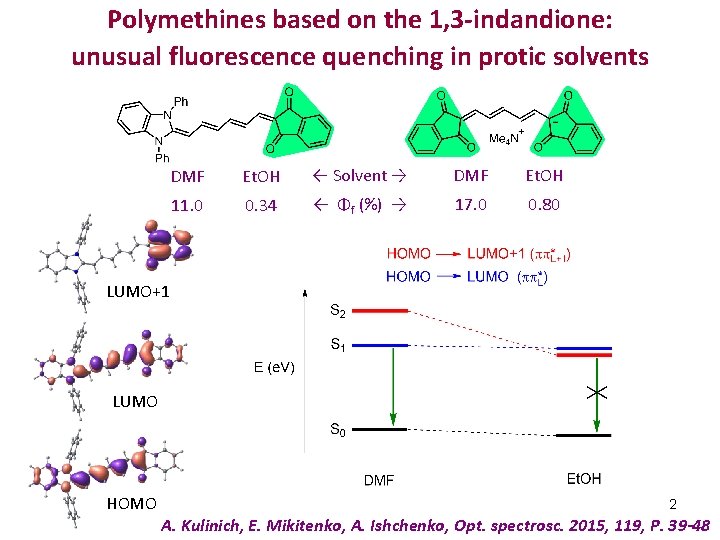
Polymethines based on the 1, 3 -indandione: unusual fluorescence quenching in protic solvents DMF Et. OH ← Solvent → DMF Et. OH 11. 0 0. 34 ← Φf (%) → 17. 0 0. 80 LUMO+1 LUMO HOMO 2 A. Kulinich, E. Mikitenko, A. Ishchenko, Opt. spectrosc. 2015, 119, P. 39 -48

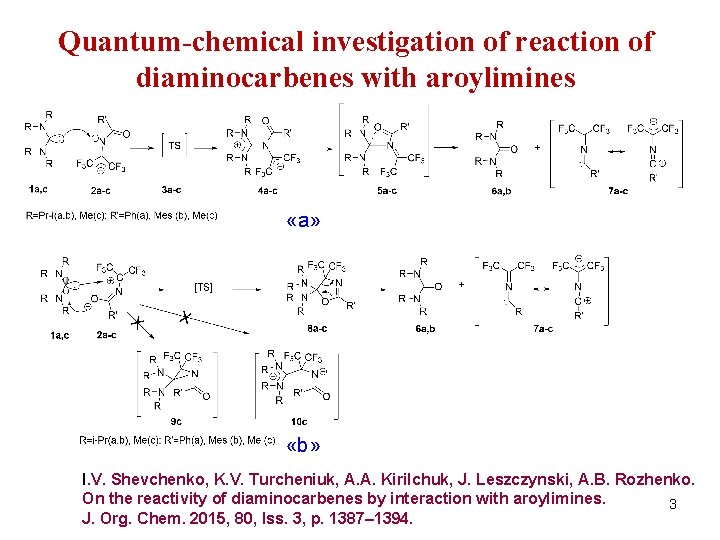
Quantum-chemical investigation of reaction of diaminocarbenes with aroylimines «a» «b» I. V. Shevchenko, K. V. Turcheniuk, A. A. Kirilchuk, J. Leszczynski, A. B. Rozhenko. On the reactivity of diaminocarbenes by interaction with aroylimines. 3 J. Org. Chem. 2015, 80, Iss. 3, p. 1387– 1394.

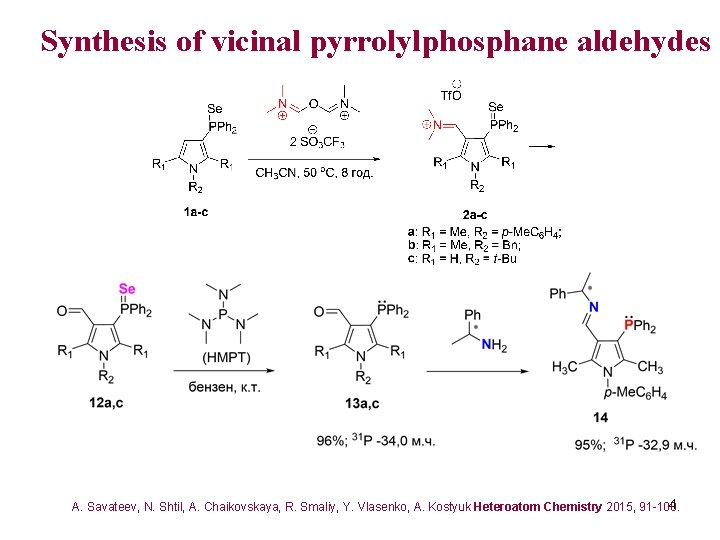
Synthesis of vicinal pyrrolylphosphane aldehydes 4 A. Savateev, N. Shtil, A. Chaikovskaya, R. Smaliy, Y. Vlasenko, A. Kostyuk Heteroatom Chemistry 2015, 91 -100.

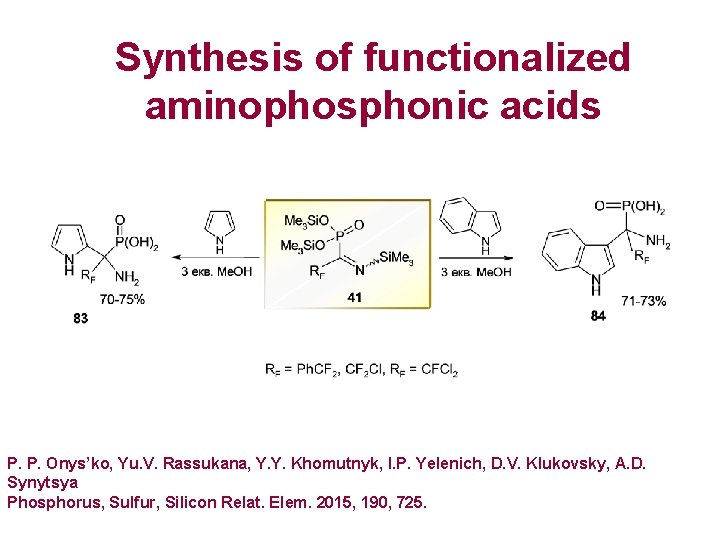
Synthesis of functionalized aminophosphonic acids P. P. Onys’ko, Yu. V. Rassukana, Y. Y. Khomutnyk, I. P. Yelenich, D. V. Klukovsky, A. D. Synytsya Phosphorus, Sulfur, Silicon Relat. Elem. 2015, 190, 725.

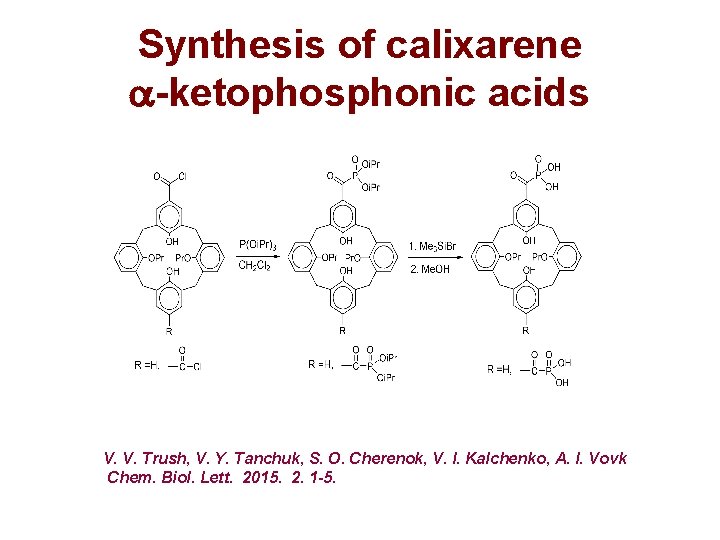
Synthesis of calixarene -ketophosphonic acids V. V. Trush, V. Y. Tanchuk, S. O. Cherenok, V. I. Kalchenko, A. I. Vovk Chem. Biol. Lett. 2015. 2. 1 -5.

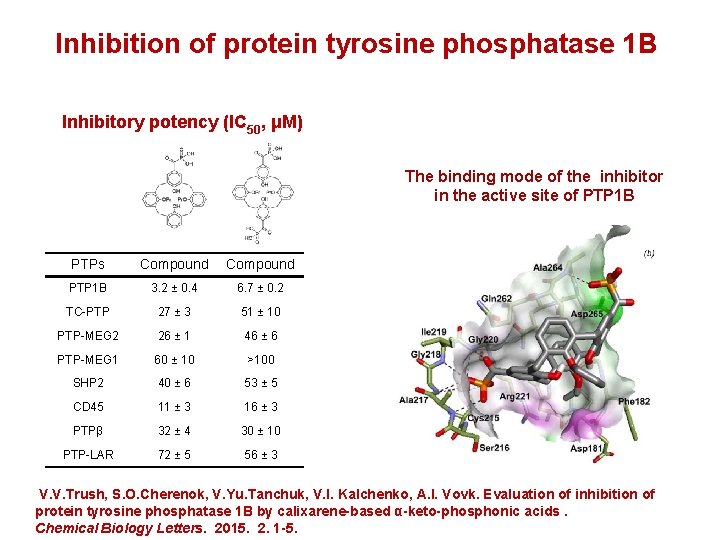
Inhibition of protein tyrosine phosphatase 1 B Inhibitory potency (IC 50, μM) The binding mode of the inhibitor in the active site of PTP 1 B PTPs Compound PTP 1 B 3. 2 ± 0. 4 6. 7 ± 0. 2 TC-PTP 27 ± 3 51 ± 10 PTP-MEG 2 26 ± 1 46 ± 6 PTP-MEG 1 60 ± 10 >100 SHP 2 40 ± 6 53 ± 5 CD 45 11 ± 3 16 ± 3 РТРβ 32 ± 4 30 ± 10 PTP-LAR 72 ± 5 56 ± 3 V. V. Trush, S. O. Cherenok, V. Yu. Tanchuk, V. I. Kalchenko, A. I. Vovk. Evaluation of inhibition of protein tyrosine phosphatase 1 B by calixarene-based α-keto-phosphonic acids. Chemical Biology Letters. 2015. 2. 1 -5.

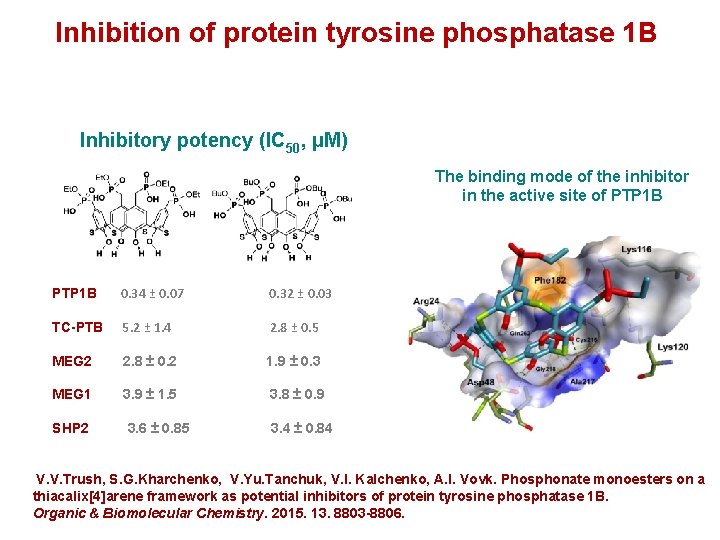
Inhibition of protein tyrosine phosphatase 1 B Inhibitory potency (IC 50, μM) The binding mode of the inhibitor in the active site of PTP 1 B 0. 34 ± 0. 07 0. 32 ± 0. 03 TC-PTB 5. 2 ± 1. 4 2. 8 ± 0. 5 MEG 2 2. 8 ± 0. 2 1. 9 ± 0. 3 MEG 1 3. 9 ± 1. 5 3. 8 ± 0. 9 SHP 2 3. 6 ± 0. 85 3. 4 ± 0. 84 V. V. Trush, S. G. Kharchenko, V. Yu. Tanchuk, V. I. Kalchenko, A. I. Vovk. Phosphonate monoesters on a thiacalix[4]arene framework as potential inhibitors of protein tyrosine phosphatase 1 B. Organic & Biomolecular Chemistry. 2015. 13. 8803 -8806.

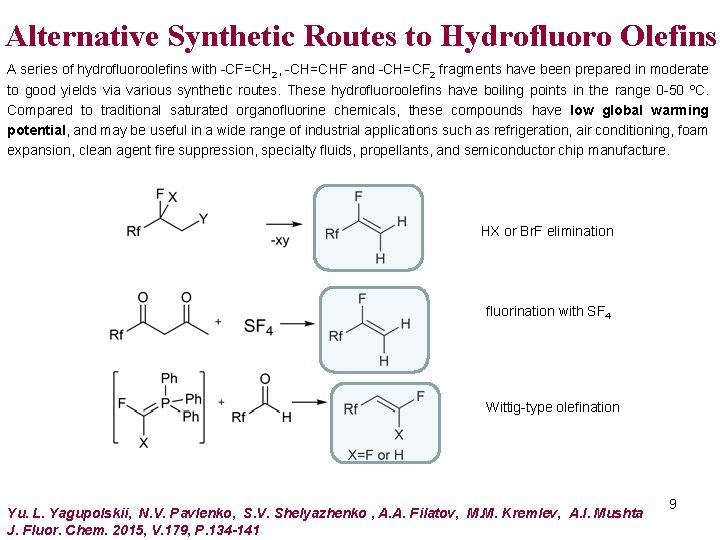
Alternative Synthetic Routes to Hydrofluoro Olefins A series of hydrofluoroolefins with -CF=CH 2, -CH=CHF and -CH=CF 2 fragments have been prepared in moderate to good yields via various synthetic routes. These hydrofluoroolefins have boiling points in the range 0 -50 ºC. Compared to traditional saturated organofluorine chemicals, these compounds have low global warming potential, and may be useful in a wide range of industrial applications such as refrigeration, air conditioning, foam expansion, clean agent fire suppression, specialty fluids, propellants, and semiconductor chip manufacture. HX or Br. F elimination fluorination with SF 4 Wittig-type olefination Yu. L. Yagupolskii, N. V. Pavlenko, S. V. Shelyazhenko , A. A. Filatov, M. M. Kremlev, A. I. Mushta J. Fluor. Chem. 2015, V. 179, P. 134 -141 9

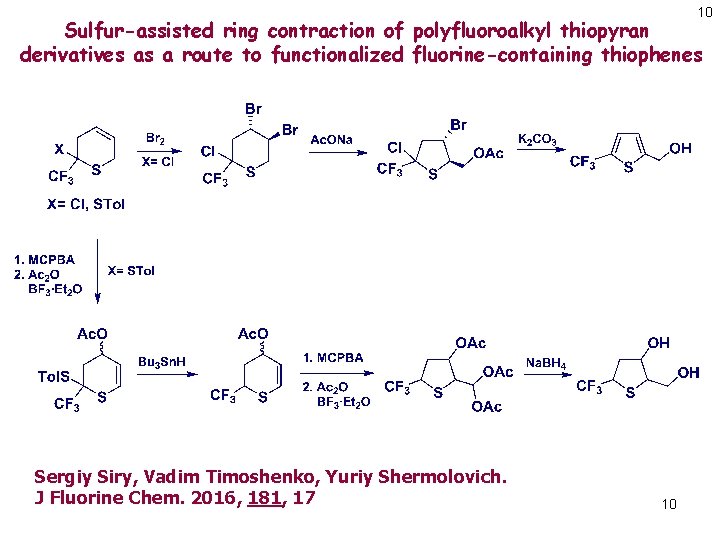
10 Sulfur-assisted ring contraction of polyfluoroalkyl thiopyran derivatives as a route to functionalized fluorine-containing thiophenes Sergiy Siry, Vadim Timoshenko, Yuriy Shermolovich. J Fluorine Chem. 2016, 181, 17 10

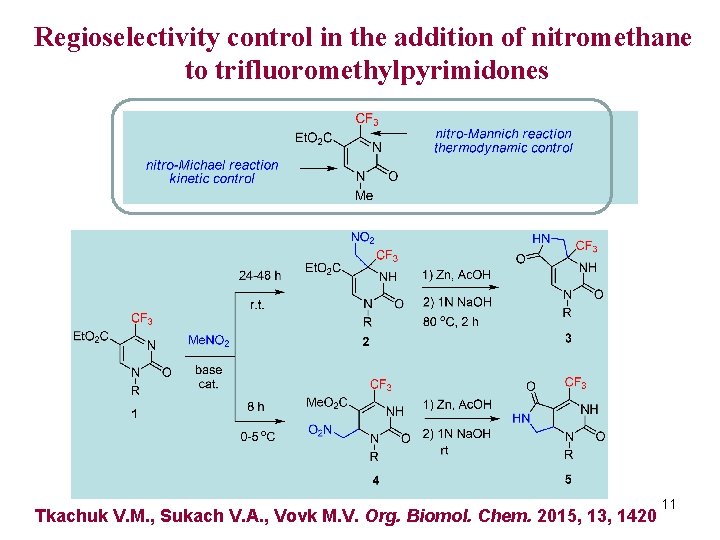
Regioselectivity control in the addition of nitromethane to trifluoromethylpyrimidones Tkachuk V. M. , Sukach V. A. , Vovk M. V. Org. Biomol. Chem. 2015, 13, 1420 11
![2 6 DISUBSTITUTED SPIRO3 3HEPTANES Chernykh A V Volochnyuk D M Journal of 2, 6 -DISUBSTITUTED SPIRO[3. 3]HEPTANES Chernykh, A. V. ; Volochnyuk D. M. Journal of](https://slidetodoc.com/presentation_image_h2/5f97f5cf1feb81e59ccd4a14bcfc607a/image-12.jpg)
2, 6 -DISUBSTITUTED SPIRO[3. 3]HEPTANES Chernykh, A. V. ; Volochnyuk D. M. Journal of Organic Chemistry, 2015, 80, 3974 -398112
![New heterocyclic system of pyrrolo3 4 d1 2diazepine pyrrole analogues of antagonists of AMPAreceptor New heterocyclic system of pyrrolo[3, 4 -d][1, 2]diazepine pyrrole analogues of antagonists of AMPA-receptor](https://slidetodoc.com/presentation_image_h2/5f97f5cf1feb81e59ccd4a14bcfc607a/image-13.jpg)
New heterocyclic system of pyrrolo[3, 4 -d][1, 2]diazepine pyrrole analogues of antagonists of AMPA-receptor Bondarenko O. , Nikolaev O. , Borodkin Ya. , Bogza S.

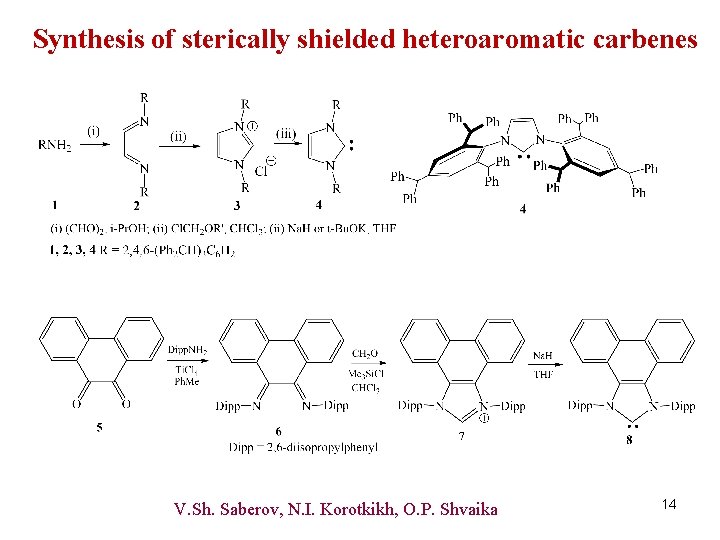
Synthesis of sterically shielded heteroaromatic carbenes V. Sh. Saberov, N. I. Korotkikh, O. P. Shvaika 14
 Nuclear safety institute of the russian academy of sciences
Nuclear safety institute of the russian academy of sciences Nars railroad
Nars railroad National academy of sciences forensic science
National academy of sciences forensic science Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Human sciences vs natural sciences tok
Human sciences vs natural sciences tok Symbiosis hospital management
Symbiosis hospital management Danish institute of agricultural sciences
Danish institute of agricultural sciences Health and environmental sciences institute
Health and environmental sciences institute Institute for telecommunication sciences
Institute for telecommunication sciences Kafue institute of health sciences
Kafue institute of health sciences Kafue institute of health sciences
Kafue institute of health sciences Hazleton area academy of sciences
Hazleton area academy of sciences Academy of motion picture arts and sciences benefits
Academy of motion picture arts and sciences benefits Hawaii academy of arts and sciences
Hawaii academy of arts and sciences



























