Impurities Defects Continued More on Shallow Donors Acceptors






- Slides: 24

Impurities & Defects, Continued More on Shallow Donors & Acceptors

More on Shallow Donors & Acceptors • As we’ve discussed, usually, semiconductors can relatively easily be doped with shallow donors and/or acceptors. • Doping Incorporation of (substitutional) impurities into a semiconductor in order for it to have certain desired electrical (or other) properties. That is, doping is the process of purposely introducing impurities into the material in a controlled manner. This is done because Impurities can drastically alter the material conductivity so that it can be useful in a device.

Impurities can drastically alter the material conductivity so that it can be useful in a device. • Materials which have been doped in this way are called Extrinsic Semiconductors. • We’ve already discussed the fact that impurity atoms can be either Substitutional Impurities: Foreign atoms occupying host atom sites. OR Interstitial Impurities: Foreign atoms “squeezed” between lattice sites. • Here, we focus on substitutional impurities only!

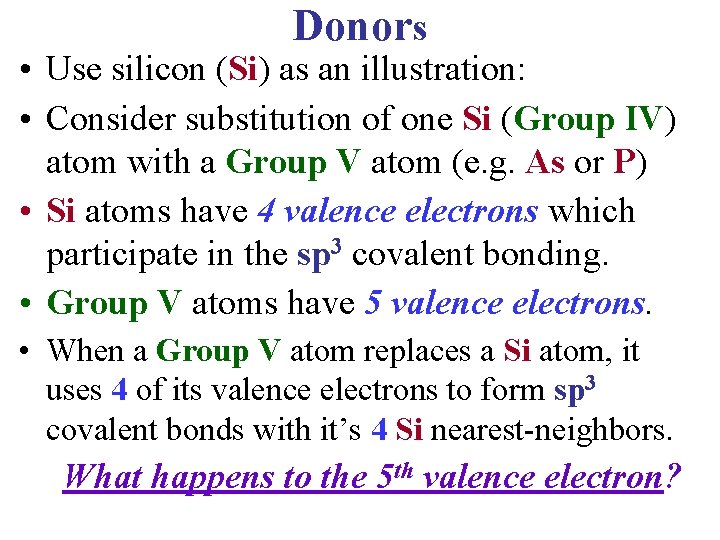
Donors • Use silicon (Si) as an illustration: • Consider substitution of one Si (Group IV) atom with a Group V atom (e. g. As or P) • Si atoms have 4 valence electrons which participate in the sp 3 covalent bonding. • Group V atoms have 5 valence electrons. • When a Group V atom replaces a Si atom, it uses 4 of its valence electrons to form sp 3 covalent bonds with it’s 4 Si nearest-neighbors. What happens to the 5 th valence electron?

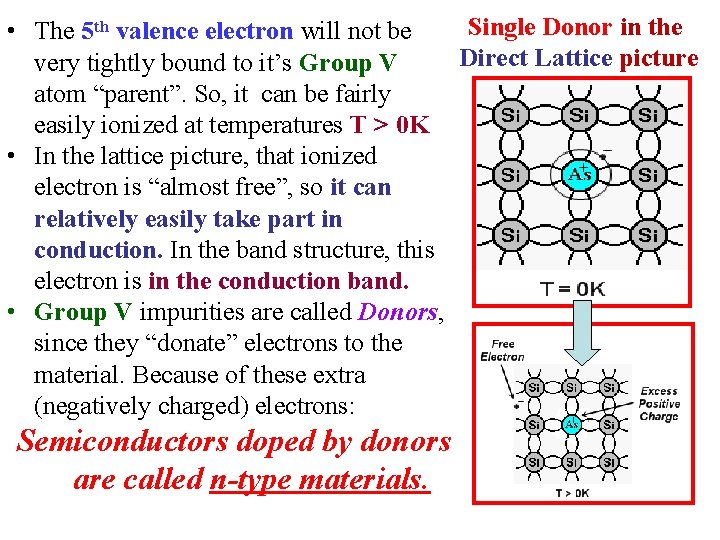
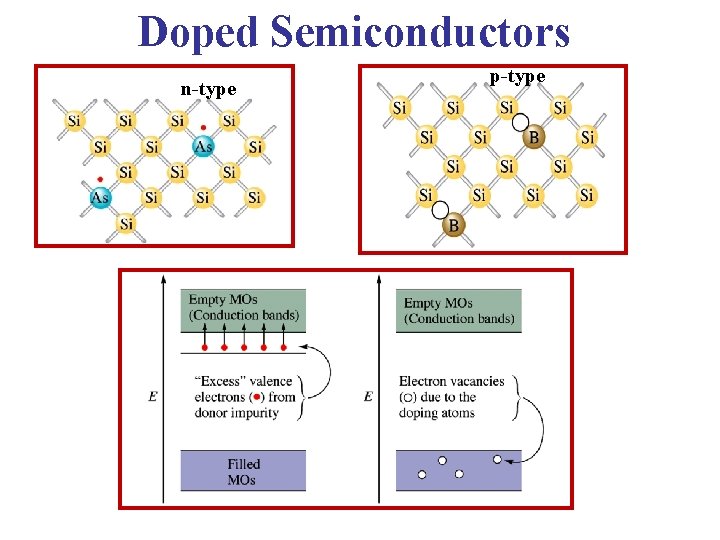
Single Donor in the • The 5 th valence electron will not be Direct Lattice picture very tightly bound to it’s Group V atom “parent”. So, it can be fairly easily ionized at temperatures T > 0 K • In the lattice picture, that ionized electron is “almost free”, so it can relatively easily take part in conduction. In the band structure, this electron is in the conduction band. • Group V impurities are called Donors, since they “donate” electrons to the material. Because of these extra (negatively charged) electrons: Semiconductors doped by donors are called n-type materials.

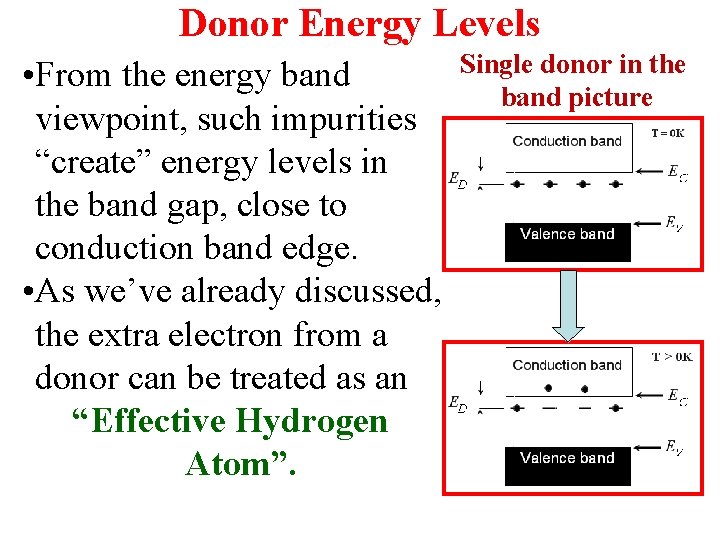
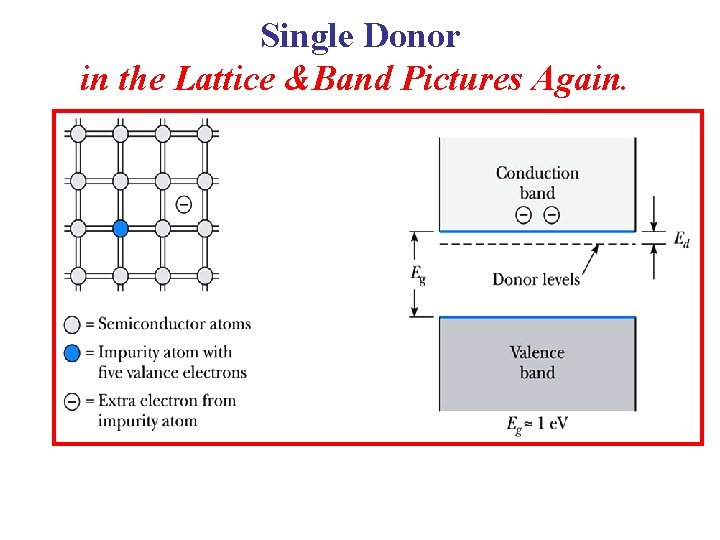
Donor Energy Levels • From the energy band viewpoint, such impurities “create” energy levels in the band gap, close to conduction band edge. • As we’ve already discussed, the extra electron from a donor can be treated as an “Effective Hydrogen Atom”. Single donor in the band picture

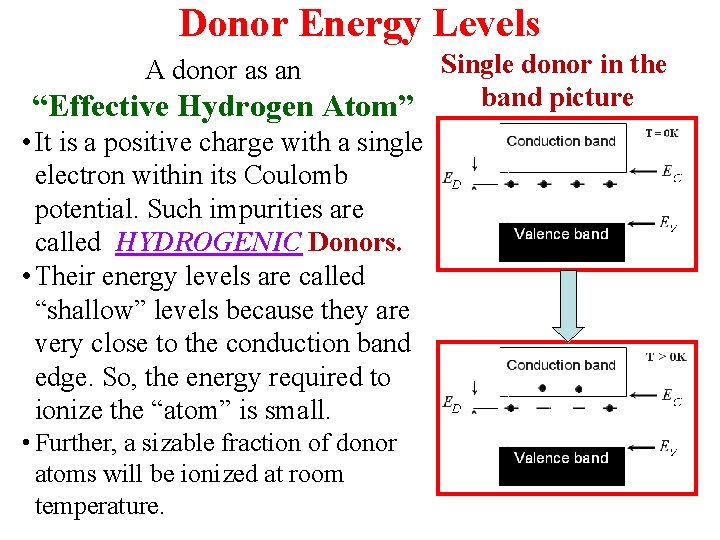
Donor Energy Levels Single donor in the band picture “Effective Hydrogen Atom” • It is a positive charge with a single electron within its Coulomb potential. Such impurities are called HYDROGENIC Donors. • Their energy levels are called “shallow” levels because they are very close to the conduction band edge. So, the energy required to ionize the “atom” is small. A donor as an • Further, a sizable fraction of donor atoms will be ionized at room temperature.

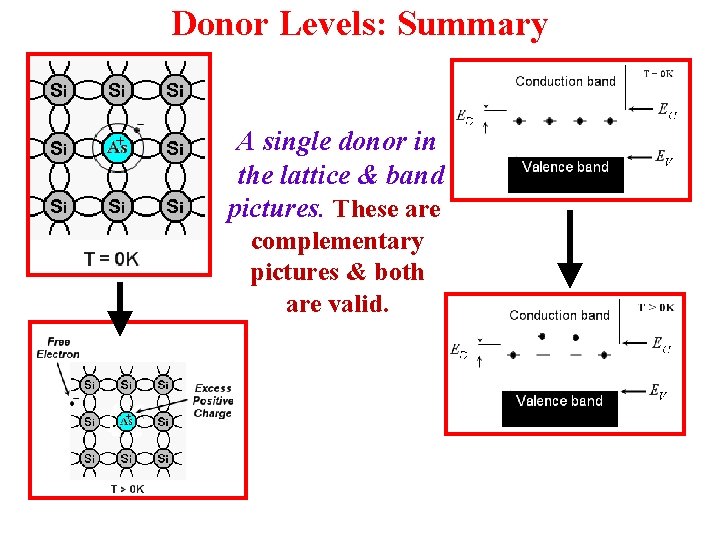
Donor Levels: Summary A single donor in the lattice & band pictures. These are complementary pictures & both are valid.

Single Donor in the Lattice &Band Pictures Again.

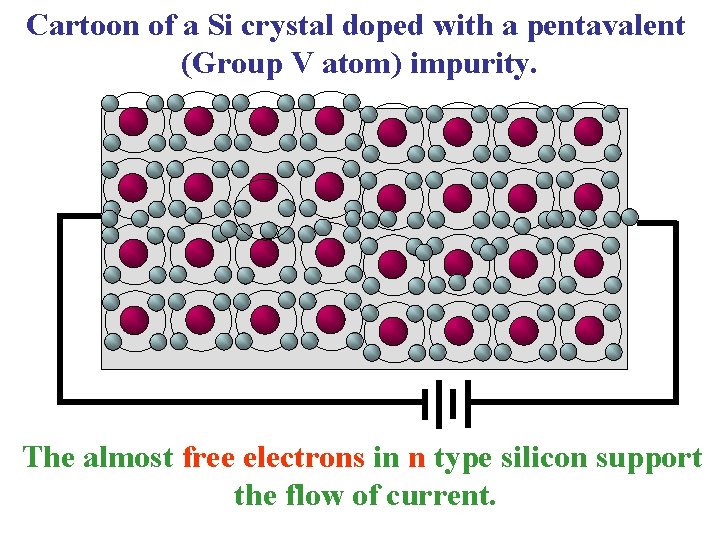
Cartoon of a Si crystal doped with a pentavalent (Group V atom) impurity. The almost free electrons in n type silicon support the flow of current.

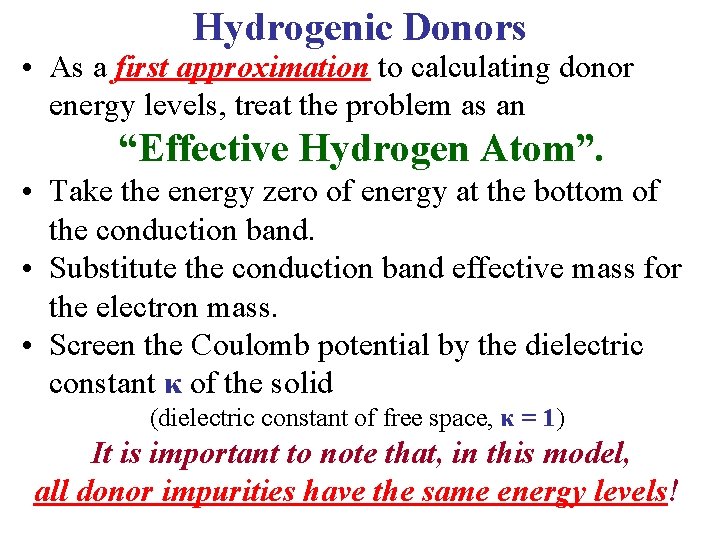
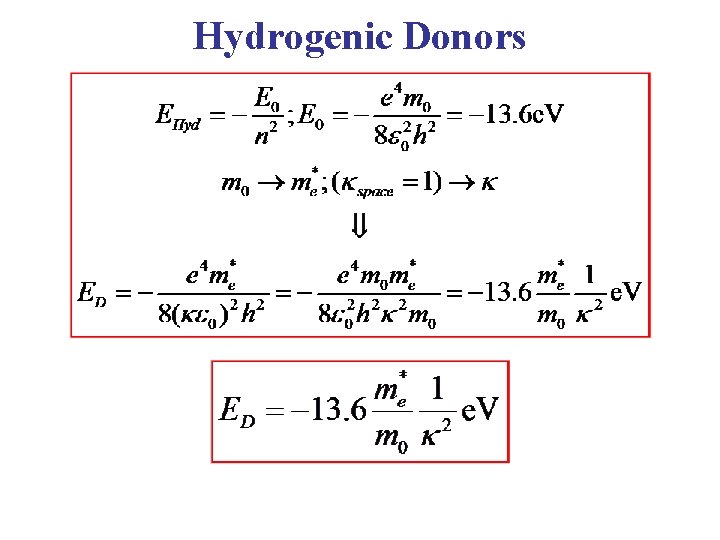
Hydrogenic Donors • As a first approximation to calculating donor energy levels, treat the problem as an “Effective Hydrogen Atom”. • Take the energy zero of energy at the bottom of the conduction band. • Substitute the conduction band effective mass for the electron mass. • Screen the Coulomb potential by the dielectric constant κ of the solid (dielectric constant of free space, κ = 1) It is important to note that, in this model, all donor impurities have the same energy levels!

Hydrogenic Donors

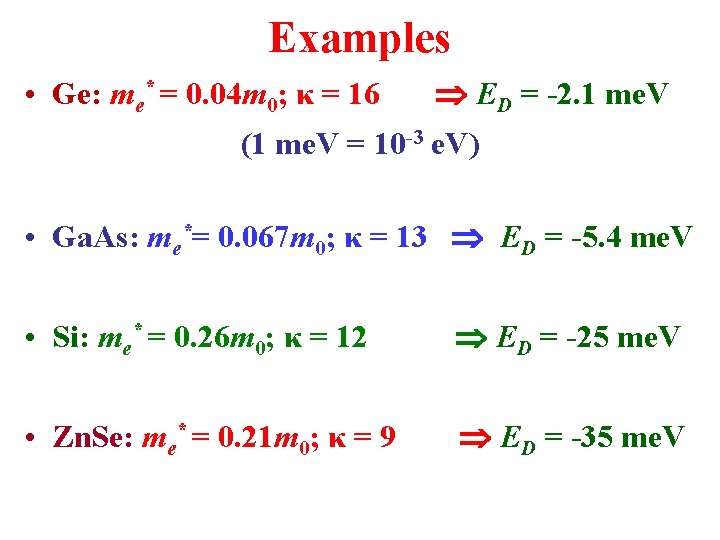
Examples • Ge: me* = 0. 04 m 0; κ = 16 ED = -2. 1 me. V (1 me. V = 10 -3 e. V) • Ga. As: me*= 0. 067 m 0; κ = 13 ED = -5. 4 me. V • Si: me* = 0. 26 m 0; κ = 12 ED = -25 me. V • Zn. Se: me* = 0. 21 m 0; κ = 9 ED = -35 me. V

Acceptors • Again use silicon (Si) as an example • Substitute one Group III atom (e. g. Al or In) for a Si (Group IV) atom • Si atoms have 4 valence electrons that participate in the covalent bonding • When a Group III atom replaces a Si atom, it cannot complete a tetravalent bond scheme.

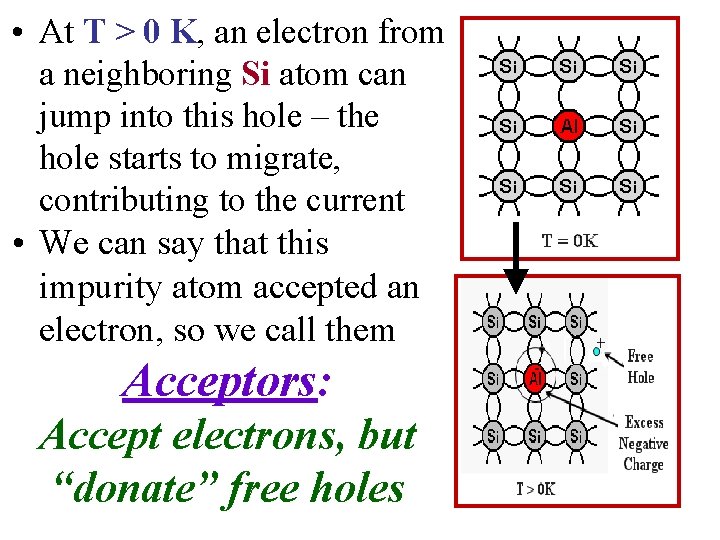
Acceptors • When a Group III atom replaces a Si atom, it cannot complete a tetravalent bond scheme. • An “electronic vacancy” – hole – is formed when an electron from the valence band is grabbed by the atom so that the core is negatively charged, the hole created is then attracted to the negative core • At T = 0 K this hole “stays” with atom – localized hole • At T > 0 K, electron from the neighboring Si atom can jump into this hole – the hole can then migrate & contribute to the conductivity.

• At T > 0 K, an electron from a neighboring Si atom can jump into this hole – the hole starts to migrate, contributing to the current • We can say that this impurity atom accepted an electron, so we call them Acceptors: Accept electrons, but “donate” free holes

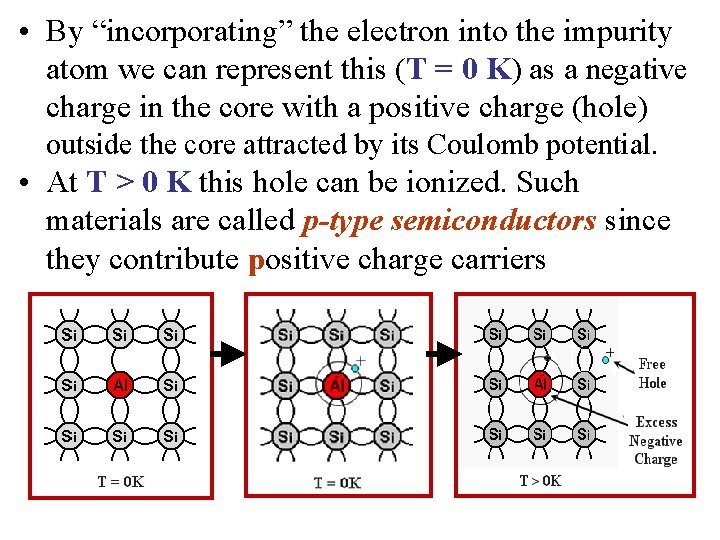
• By “incorporating” the electron into the impurity atom we can represent this (T = 0 K) as a negative charge in the core with a positive charge (hole) outside the core attracted by its Coulomb potential. • At T > 0 K this hole can be ionized. Such materials are called p-type semiconductors since they contribute positive charge carriers

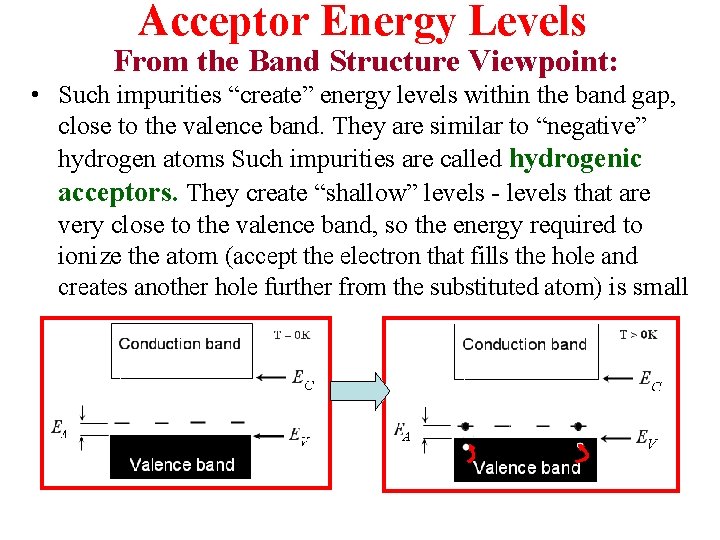
Acceptor Energy Levels From the Band Structure Viewpoint: • Such impurities “create” energy levels within the band gap, close to the valence band. They are similar to “negative” hydrogen atoms Such impurities are called hydrogenic acceptors. They create “shallow” levels - levels that are very close to the valence band, so the energy required to ionize the atom (accept the electron that fills the hole and creates another hole further from the substituted atom) is small

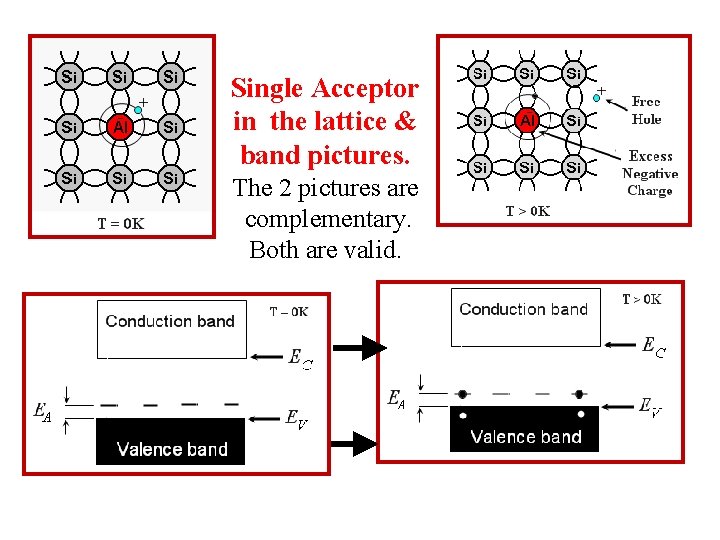
Single Acceptor in the lattice & band pictures. The 2 pictures are complementary. Both are valid.

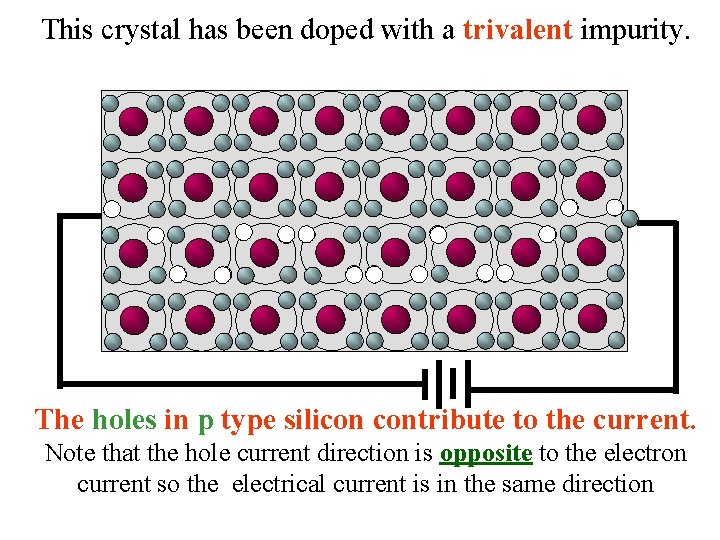
This crystal has been doped with a trivalent impurity. The holes in p type silicon contribute to the current. Note that the hole current direction is opposite to the electron current so the electrical current is in the same direction


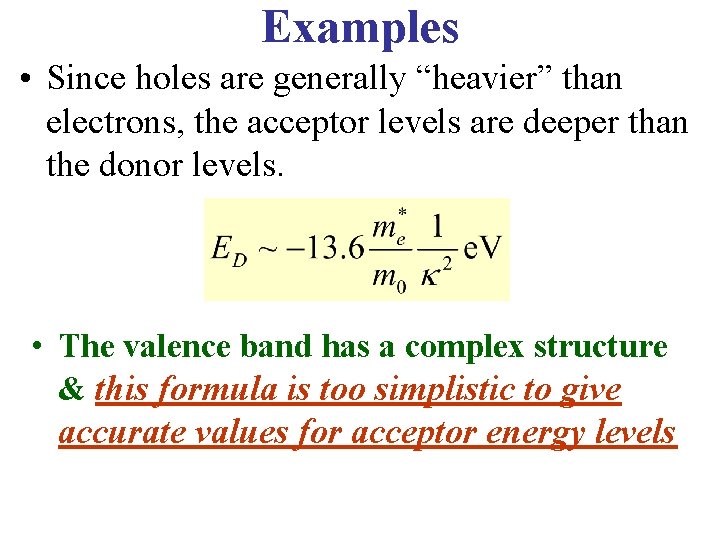
Examples • Since holes are generally “heavier” than electrons, the acceptor levels are deeper than the donor levels. • The valence band has a complex structure & this formula is too simplistic to give accurate values for acceptor energy levels

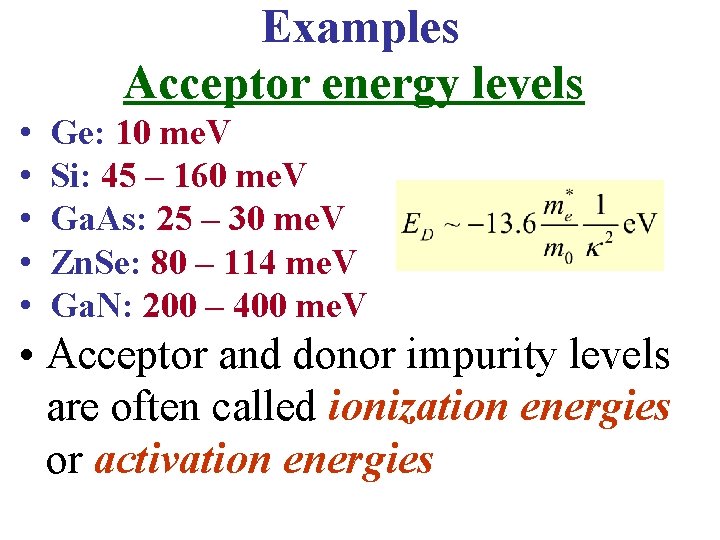
Examples Acceptor energy levels • • • Ge: 10 me. V Si: 45 – 160 me. V Ga. As: 25 – 30 me. V Zn. Se: 80 – 114 me. V Ga. N: 200 – 400 me. V • Acceptor and donor impurity levels are often called ionization energies or activation energies

Doped Semiconductors n-type p-type