Immunosuppression Prof Ileana Constantinescu Immunosuppression Immunosuppressive protocols in




















- Slides: 30

Immunosuppression Prof. Ileana Constantinescu

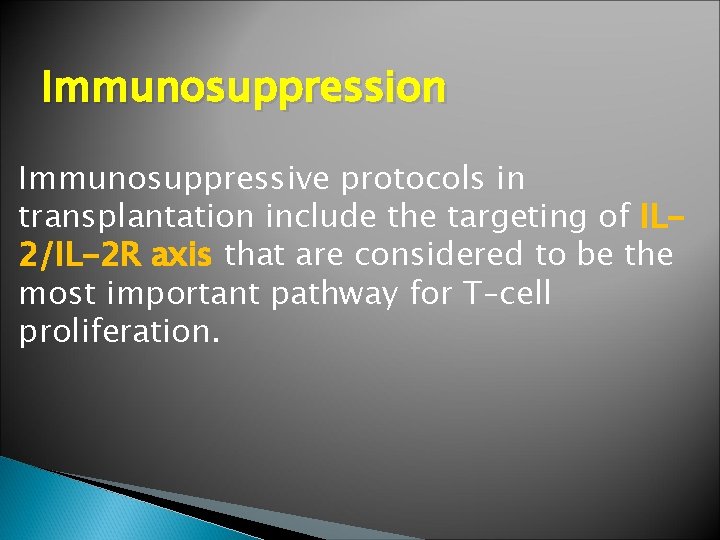
Immunosuppression Immunosuppressive protocols in transplantation include the targeting of IL 2/IL-2 R axis that are considered to be the most important pathway for T–cell proliferation.

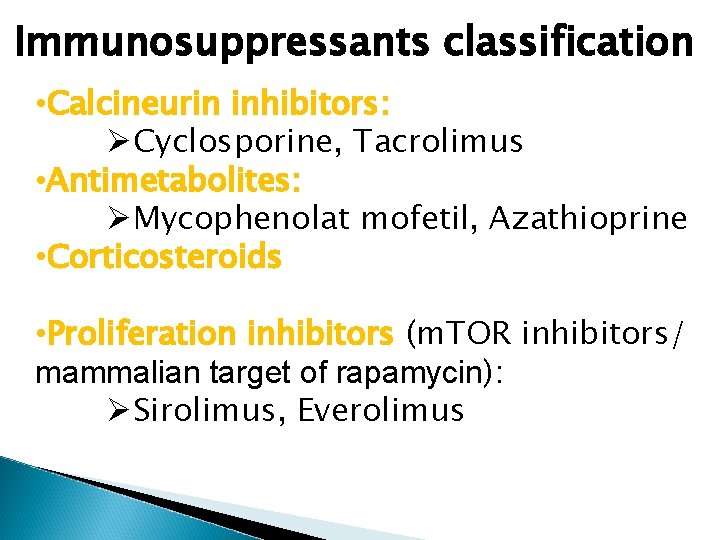
Immunosuppressants classification • Calcineurin inhibitors: ØCyclosporine, Tacrolimus • Antimetabolites: ØMycophenolat mofetil, Azathioprine • Corticosteroids • Proliferation inhibitors (m. TOR inhibitors/ mammalian target of rapamycin): ØSirolimus, Everolimus


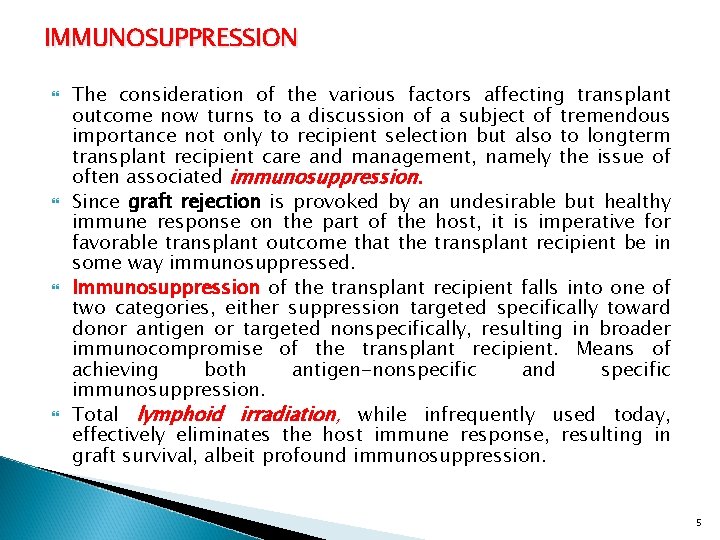
IMMUNOSUPPRESSION The consideration of the various factors affecting transplant outcome now turns to a discussion of a subject of tremendous importance not only to recipient selection but also to longterm transplant recipient care and management, namely the issue of often associated immunosuppression. Since graft rejection is provoked by an undesirable but healthy immune response on the part of the host, it is imperative for favorable transplant outcome that the transplant recipient be in some way immunosuppressed. Immunosuppression of the transplant recipient falls into one of two categories, either suppression targeted specifically toward donor antigen or targeted nonspecifically, resulting in broader immunocompromise of the transplant recipient. Means of achieving both antigen-nonspecific and specific immunosuppression. Total lymphoid irradiation, while infrequently used today, effectively eliminates the host immune response, resulting in graft survival, albeit profound immunosuppression. 5

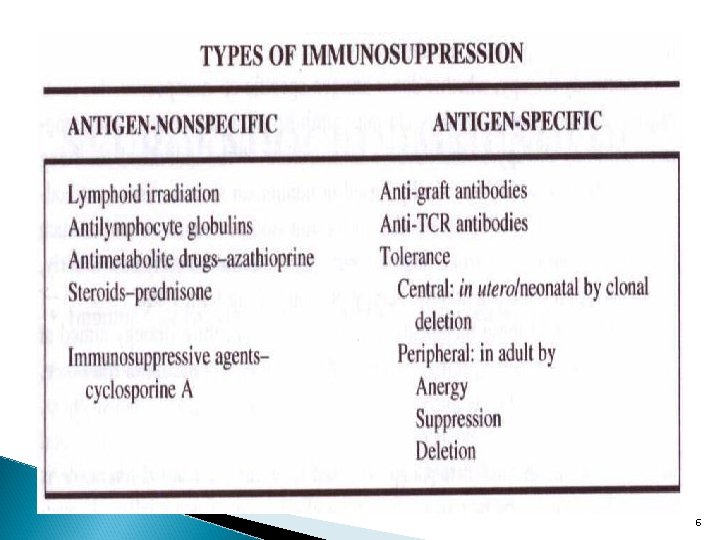
6

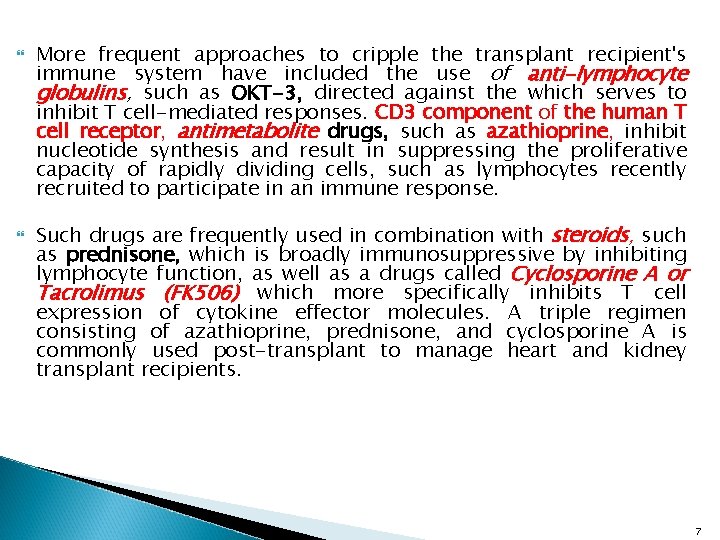
More frequent approaches to cripple the transplant recipient's immune system have included the use of anti-lymphocyte globulins, such as OKT-3, directed against the which serves to inhibit T cell-mediated responses. CD 3 component of the human T cell receptor, antimetabolite drugs, such as azathioprine, inhibit nucleotide synthesis and result in suppressing the proliferative capacity of rapidly dividing cells, such as lymphocytes recently recruited to participate in an immune response. Such drugs are frequently used in combination with steroids, such as prednisone, which is broadly immunosuppressive by inhibiting lymphocyte function, as well as a drugs called Cyclosporine A or Tacrolimus (FK 506) which more specifically inhibits T cell expression of cytokine effector molecules. A triple regimen consisting of azathioprine, prednisone, and cyclosporine A is commonly used post-transplant to manage heart and kidney transplant recipients. 7

Such effective immunosuppressive measures, due to their broad impact on the host's immune system, as well as frequent adverse side effects, have raised the issue of identifying means of suppressing the transplant rejection response in a more antigen-specific way. It is hoped that such antigen-specific means of immunosuppression, though still in their infancy, will impact less on the host's immune system and result in fewer side effects associated with multiple drug use. One strategy considered in antigen-specific immunosuppression is to employ antibodies directed against graft antigens, such as MHC alloantigens, which results in a prolongation of graft survival known as enhancement. The mechanism by which anti-graft antibodies promote transplant survival is currently under investigation. Another donor antigen-specific approach to immunsuppression, still in an experimental phase of study, is the use of anti-T cell receptor (TCR) antibodies to block host T cells specific for allogeneic MHC.

This kind of therapy implies a detailed knowledge not only of which MHC alloantigens are expressed on each graft, but also which antigen epitopes are critical for generating host T cell help during the alloimmune response. Antibody therapy, whether donor antigen-specific or nonspecific, due to its passive nature, usually requires chronic administration. Associated with long-term antibody therapy is the adverse sensitization of the individual receiving these antibodies, which are usually raised in nonhuman species. These antibodies, therefore, act as alloantigens due to foreign isotope sequences and as such have the potential to provoke anti-antibody responses in the recipient. Clearly, such a situation renders antibody therapy of limited longterm value. 9

In the face of these therapeutic difficulties, an appealing strategy aimed at inducing donor antigen specific immunosuppression is the concept of tolerance, defined here as specific, acquired, long lasting immunological unresponsiveness. Tolerance to self antigens occurs very early in life, during fetal and neonatal development. Self-tolerance or central tolerance is principally acquired by negative selection or elimination of potentially self-reactive T cell clones during thymic development. Negative selection in the thymus is characterized by programmed cell death in a process referred to as clonal deletion. Tolerance to prospective donor alloantigens, however, raises the issue of how to achieve tolerance in the adult transplant recipient long after central or selftolerance has been achieved. Issues surrounding how to establish peripheral (nonthymic) tolerance form the basis of many experimental studies in the fields of transplantation and autoimmunity today. While still at their inception, these studies aim to elucidate how prior oral or intravenous administration of donor allo MHC antigens, in the case of transplantation, sometimes achieves a tolerant state in the transplant recipient. 10

Also under investigation are the doses of antigen required to induce tolerance, described as either low zone or high zone, depending upon the relative doses of antigen needed to achieve it. Route of administration and antigen dose aside, experimental observations published to date have indicated that tolerance to donor allo MHC antigens may be established by a combination of active suppression of the recipient immune response and/or failure to respond to foreign antigen mediated by either T cell clonal deletion or anergy. Anergy may be defined as the absence of an immune response due to loss of cell function or "immune paralysis" and may be reversible. Future transplant therapy awaits clarification of these issues.

Immunosupression Cyclosporin A: MEIA Axsym/TDx, HPLC Tacrolimus: MEIA IMx, HPLC Sirolimus: MEIA IMx, HPLC Methotrexat: MEIA TDx






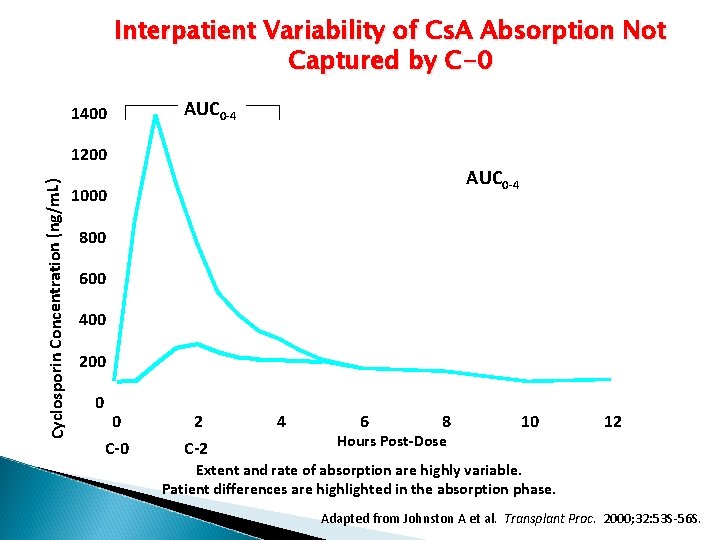
Interpatient Variability of Cs. A Absorption Not Captured by C-0 1400 AUC 0 -4 Cyclosporin Concentration (ng/m. L) 1200 AUC 0 -4 1000 800 600 400 200 0 0 C-0 2 C-2 4 6 8 Hours Post-Dose 10 12 Extent and rate of absorption are highly variable. Patient differences are highlighted in the absorption phase. Adapted from Johnston A et al. Transplant Proc. 2000; 32: 53 S-56 S.






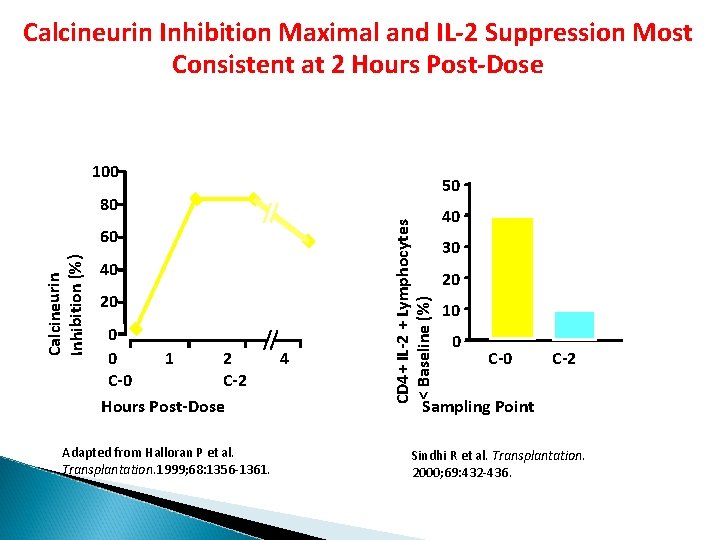
Calcineurin Inhibition Maximal and IL-2 Suppression Most Consistent at 2 Hours Post-Dose 100 50 Calcineurin Inhibition (%) 60 40 20 0 0 1 2 C-0 C-2 Hours Post-Dose Adapted from Halloran P et al. Transplantation. 1999; 68: 1356 -1361. 4 CD 4+ IL-2 + Lymphocytes < Baseline (%) 80 40 30 20 10 0 C-2 Sampling Point Sindhi R et al. Transplantation. 2000; 69: 432 -436.


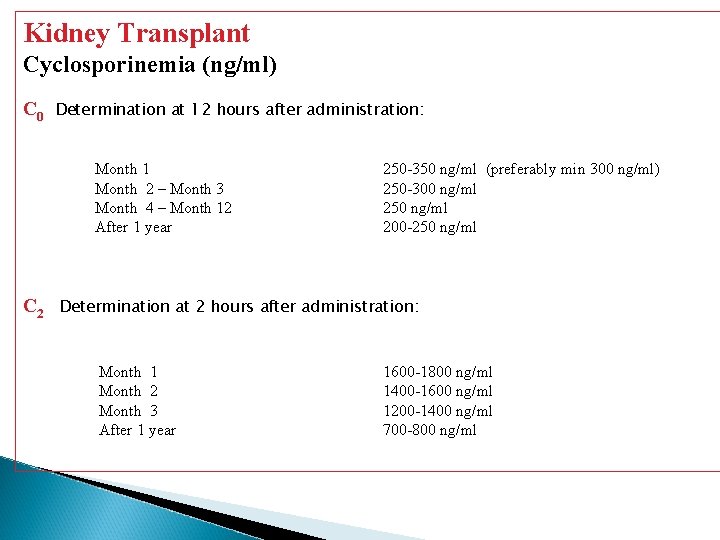
Kidney Transplant Cyclosporinemia (ng/ml) C 0 Determination at 12 hours after administration: Month 1 Month 2 – Month 3 Month 4 – Month 12 After 1 year C 2 250 -350 ng/ml (preferably min 300 ng/ml) 250 -300 ng/ml 250 ng/ml 200 -250 ng/ml Determination at 2 hours after administration: Month 1 Month 2 Month 3 After 1 year 1600 -1800 ng/ml 1400 -1600 ng/ml 1200 -1400 ng/ml 700 -800 ng/ml

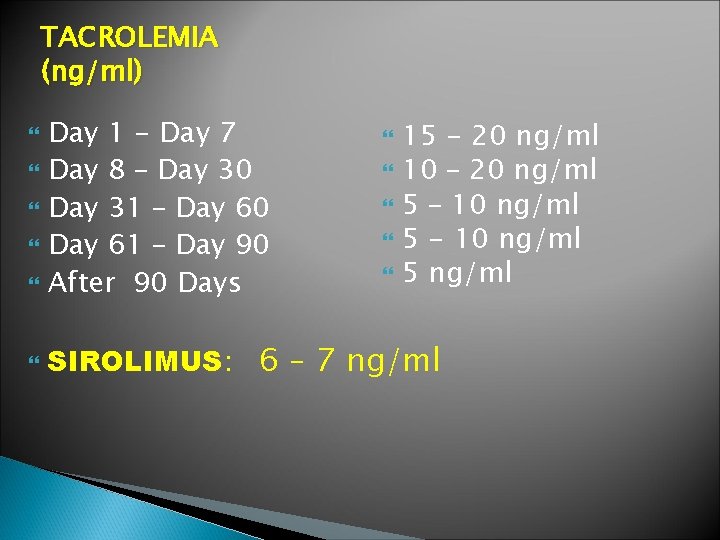
TACROLEMIA (ng/ml) Day 1 - Day 7 Day 8 – Day 30 Day 31 – Day 60 Day 61 – Day 90 After 90 Days 15 - 20 ng/ml 10 – 20 ng/ml 5 – 10 ng/ml 5 - 10 ng/ml 5 ng/ml SIROLIMUS: 6 – 7 ng/ml

Adverse reactions in immunosuppressant drugs When immunosuppressant drugs weaken the immune system, the body becomes less resistant to infection. Any infections that develop will be more difficult to treat because of this. These drugs also increase the likelihood of uncontrolled bleeding due to injury or infection. People taking immunosuppressant drugs should be careful to avoid catching an infection. Precautions include: frequent hand washing avoiding sports in which injuries occur extra care when using sharp objects such as knives or razors avoiding close contact with people who have infections or colds A physician should be notified immediately when the following symptoms occur: fever or chills pain in the lower back, on the sides pain or difficulty urinating unusual bruising or bleeding blood in your urine stools that are bloody or black

Immunosuppressant drugs can cause adverse reactions and birth defects. Physicians should be made aware of the following conditions before immunosuppressant drugs are prescribed: allergies pregnancy lactation shingles or chickenpox kidney or liver disease intestinal problems The most significant side effect of immunosuppressant drugs is an increased risk of infection. The drugs can also put you at a higher risk for cancer because the immune system also protects you from this disease. Other, less serious side effects can include loss of appetite, nausea, vomiting, increased hair growth, and hand trembling. These effects typically subside as the body adjusts to the immunosuppressant drugs. The following side effects indicate the need for immediate attention: a feeling of being unusually tired or weak fever or chills frequent urination Immunosuppressant drugs can interact with many other medications. This can cause dangerous effects in which the immunosuppressants may lose or even increase their effect. The primary caregiver should be made aware of any prescription or over-thecounter medications their patients are taking while on immunosuppressant therapy.

Conclusions Transplantation immunology is complex. Our Immunogenetic Centre provide expanded immunological monitoring together with virological and drug monitoring of the transplanted patients. Our goal is to offer complete integrated monitoring data and to fulfil EFI Standards. We are open for collaborative work and research projects within EFI. Clinical and laboratory scientists should work together as a team in order to have a complete overview of the transplanted patients.
 Ileana constantinescu fundeni
Ileana constantinescu fundeni Ileana constantinescu
Ileana constantinescu Rapamune
Rapamune Immunosuppressive drugs
Immunosuppressive drugs Immunosuppressive treatment
Immunosuppressive treatment Dr tudor constantinescu
Dr tudor constantinescu Artstico
Artstico Ileana costea
Ileana costea Ileana vulpescu
Ileana vulpescu Uml
Uml Ileana kwasinski
Ileana kwasinski Atriz ileana kwasinski
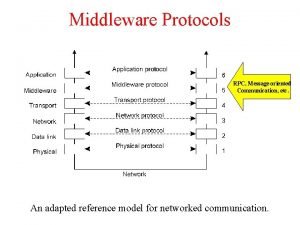
Atriz ileana kwasinski Application layer protocols
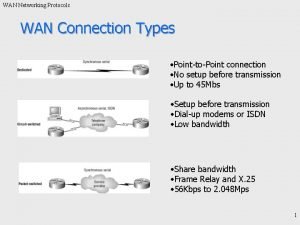
Application layer protocols Types of wan
Types of wan Sliding window protocol data link layer
Sliding window protocol data link layer Atm wan technology
Atm wan technology Accountable talk protocols
Accountable talk protocols Nyc bls protocols
Nyc bls protocols Inter vlan routing layer 3 switch
Inter vlan routing layer 3 switch Routing protocols administrative distance
Routing protocols administrative distance Internet security protocols
Internet security protocols Igmpv
Igmpv Therapist driven protocols
Therapist driven protocols Contoh aktiviti pembangunan profesionalisme berterusan
Contoh aktiviti pembangunan profesionalisme berterusan Receipt-based transient synchronous communication
Receipt-based transient synchronous communication Cryptography standards and protocols
Cryptography standards and protocols Wireless lan protocols
Wireless lan protocols 802 protocols
802 protocols Local government protocols
Local government protocols Gsm protocol
Gsm protocol Remsa emt recert
Remsa emt recert