http faculty sau edu sah alshehri Definition Proteins














- Slides: 29

http: //faculty. sau. edu. sa/h. alshehri

Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They provide support. - Act as catalysts for chemical reactions. - Carry oxygen in our blood. - Defend us from infection. - Allow movement. - Regulate cell activities

Classification: Proteins may be classified on the basis of their: - Composition - Solubility - Shape - Biological function - Three dimensional structure

I. Composition: A. Simple protein: Yields only amino acids on hydrolysis. B. Conjugated Proteins Yields amino acids and other organic and inorganic components. • Nucleoprotein (a protein containing Nuclei acids) • Lipoprotein (a protein containing lipids) • Glycoprotein (a protein containing carbohydrates) • Metalloprotein (a protein containing metals)

II. Solubility a) Albumins: These proteins such as egg albumin and serum albumin are readily soluble in water and coagulated by heat. b) Globulins: these proteins are present in serum, muscle and other tissues and are soluble in dilute salt solution but less in water. c) Histones: Histones are present in glandular tissues (thymus, pancreas etc. ) and soluble in water; they combine with nucleic acids.

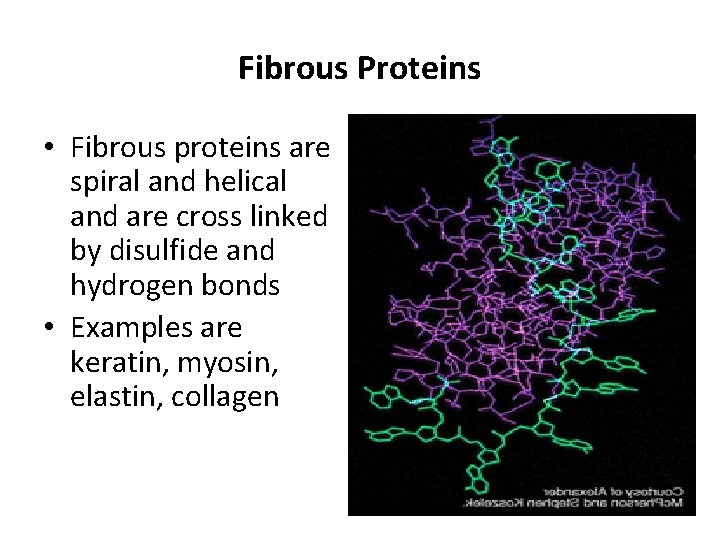
III. Shape A. Fibrous proteins: They are spiral and helical and cross-linked polypeptide chains by disulfide and hydrogen bonds, they are insoluble in water. The ratio of length to breath (axial ratio) is more than 10. Ex. Collagen, Elastin & Keratin. B. Globular proteins: These are globular in shape, and compactly folded and coiled, they are soluble in water and the axial ratio is 3 -4. Ex. Albumin, globulins and histones.

Fibrous Proteins • Fibrous proteins are spiral and helical and are cross linked by disulfide and hydrogen bonds • Examples are keratin, myosin, elastin, collagen

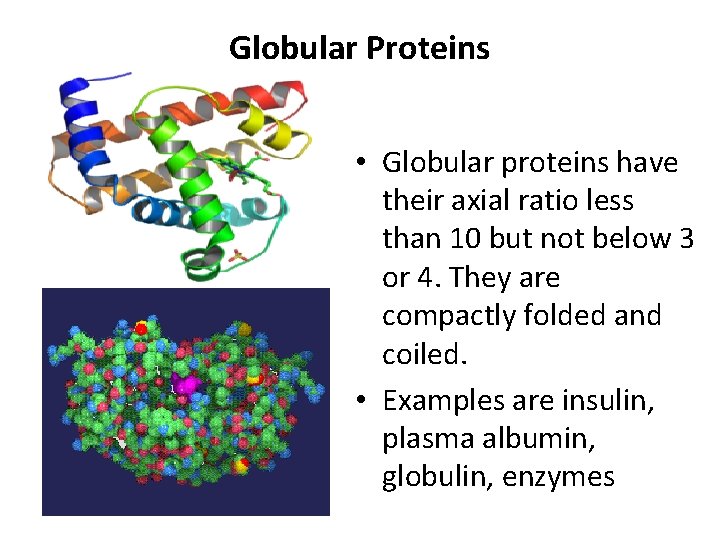
Globular Proteins • Globular proteins have their axial ratio less than 10 but not below 3 or 4. They are compactly folded and coiled. • Examples are insulin, plasma albumin, globulin, enzymes

IV. Biological Functions: • Catalytic proteins: kinases, transaminases etc. • Storage proteins: myoglobin, ferretin. • Structural protein: collagen, Proteoglycans. • Protective proteins: blood clotting factors, Immunoglobins, fibrinogen. • Transport proteins: hemoglobin, plasma lipoproteins. • Contractile or motile proteins: Actin, tubulin • Regulatory proteins: hormones.

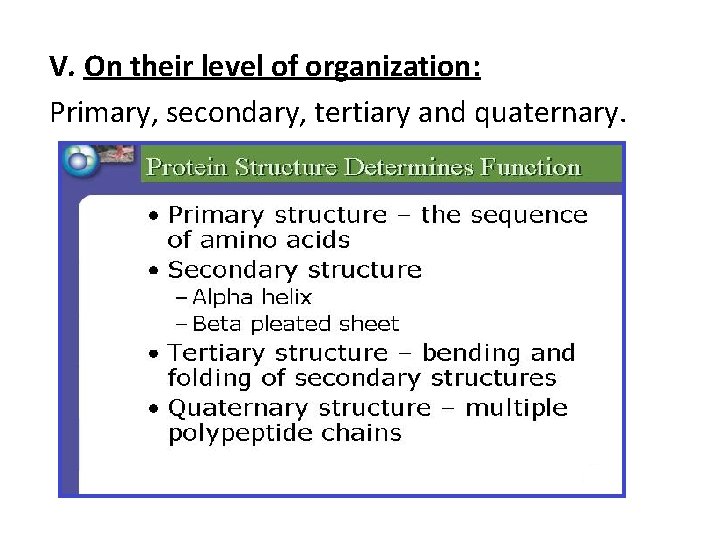
V. On their level of organization: Primary, secondary, tertiary and quaternary.



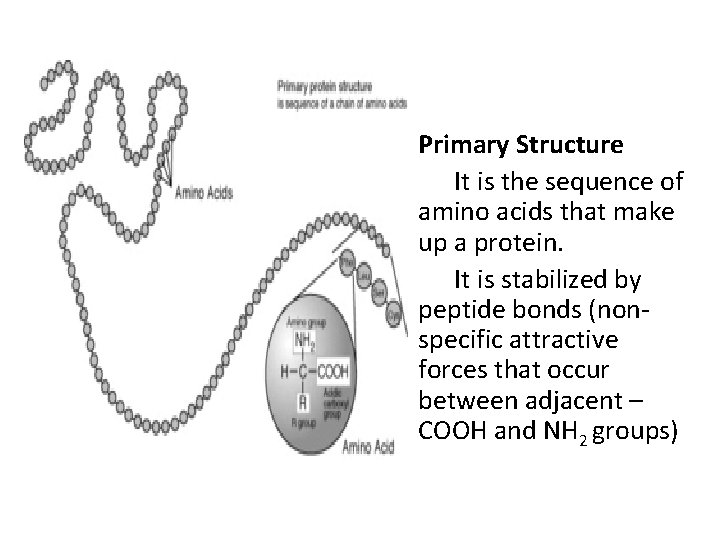
• Primary Structure It is the sequence of amino acids that make up a protein. It is stabilized by peptide bonds (nonspecific attractive forces that occur between adjacent – COOH and NH 2 groups)


Secondary structure of protein is formed by hydrogen bonding between different amino acids along the polypeptide. This leads to the formation of patterns called: • Alpha helices • Beta pleated sheets. An alpha helix is a right-handed helix found in proteins such as the keratins that are found in hair. Beta pleated sheets form when two polypeptides are lying alongside each other or a single polypeptide is bent back along itself.


Tertiary structure: Structures such as alpha helices and beta pleated sheets are commonly bent and folded together to form the tertiary structure of proteins. Tertiary structure depends on the interactions among the various R groups on the amino acids. These interactions may include: • covalent bonds • hydrophobic and van der Waals interactions • ionic bonds All these interactions serve to fold the protein into its final, functional form.


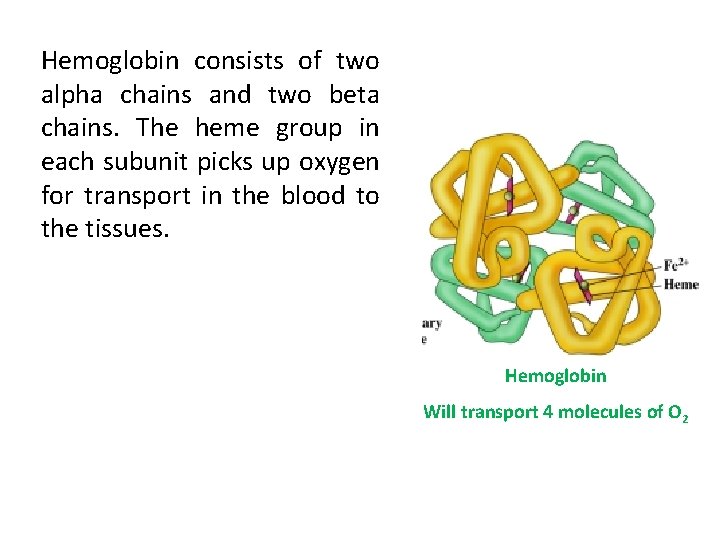
Quaternary structure It’s found in complex proteins that contain more than one polypeptide chain. For example, hemoglobin which is made up of 4 different polypeptide chains. These chains are held together by weak interactions like hydrogen bonds, van der waal forces.


Denaturation of proteins: Because the secondary, tertiary and quaternary structure of proteins depends largely on comparatively weak bonds, the higher structure of proteins is sensitive to environmental conditions such as temperature and p. H that can disrupt those bonds. For example, proteins may denature at high temperatures because hydrogen bonds and van der Waals forces become unstable at high temperatures. When proteins denature, they lose their folded shape and are unable to perform their functions. Changes in p. H can cause similar denaturation.

Denaturing agents: 1. Physical factors: temperature, pressure. 2. Chemical factors: acids and alkalis, organic solvents (acetone, ethanol), detergents (cleaning agents) and heavy metal salts (Hg, Cu, Ba, Zn) cause the denaturation.


Hydrolysis of proteins • Break down of peptide bonds. • In the lab, the hydrolysis of a peptide requires acid or base, water and heat. • In the body, enzymes like pepsin catalyze the hydrolysis of proteins. 24

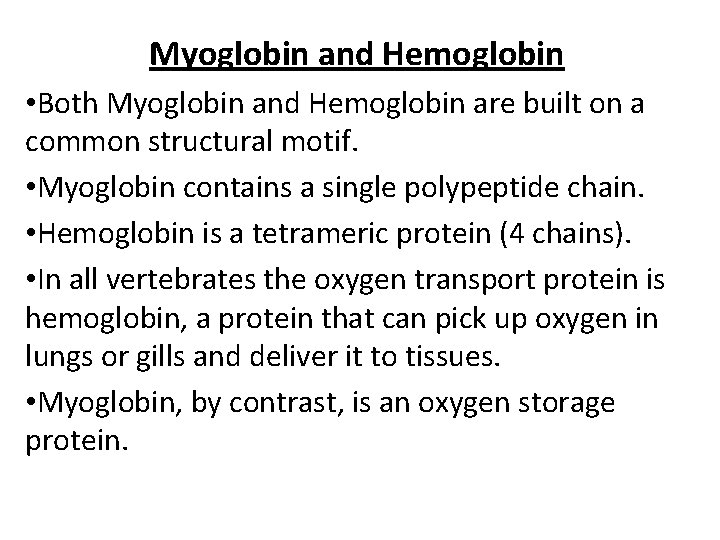
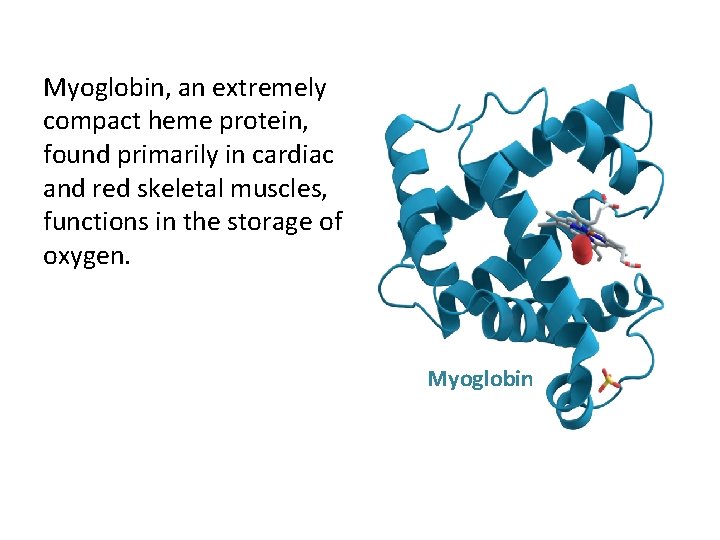
Myoglobin and Hemoglobin • Both Myoglobin and Hemoglobin are built on a common structural motif. • Myoglobin contains a single polypeptide chain. • Hemoglobin is a tetrameric protein (4 chains). • In all vertebrates the oxygen transport protein is hemoglobin, a protein that can pick up oxygen in lungs or gills and deliver it to tissues. • Myoglobin, by contrast, is an oxygen storage protein.

Myoglobin, an extremely compact heme protein, found primarily in cardiac and red skeletal muscles, functions in the storage of oxygen. Myoglobin

Hemoglobin consists of two alpha chains and two beta chains. The heme group in each subunit picks up oxygen for transport in the blood to the tissues. Hemoglobin Will transport 4 molecules of O 2

• Myoglobin (Mb) is found primarily in skeletal and striated muscle cells which mainly serves as a store of oxygen in the cytoplasm. • Hemoglobin (Hb) is restricted to the Erythrocytes which is responsible for the movement of oxygen between lungs and other tissues. • Myoglobin can bind only one molecule of oxygen because it contains only one heme group. In contrast, hemoglobin can bind four oxygen at each of its four heme groups.

• The iron in Hemoglobin or Myoglobin must be maintained in ferrous form (Fe 2+) to combine with O 2. • If the iron atom were to become oxidized to ferric form (Fe 3+), the Globin would get changed to Methemoglobin, where heme can no longer interact with O 2 and oxygen transport is blocked.