Precipitation of Proteins at isoelectric Point Proteins Proteins













- Slides: 26

Precipitation of Proteins at isoelectric Point

Proteins � Proteins are polymers consisting of 20 kinds of amino acids. � Are substance of high molecular weight from 5000 to 1000, 000 daltons. � All protein Contain C, H, O, N, and most contain sulfur, some contain phosphorus and a few have mineral elements such as Fe, Mg and Cu. � Serve as structural components of animals

Proteins

� Structure, some proteins provide structural support collagen, hair, crystallins(eyes). � Transport, some proteins are responsible for the transportation of smaller molecules from one part of the body to another, transport across cell membranes, etc. An example is hemoglobin, which transports oxygen from the lungs to cells throughout the body.

� Catalysis enzymes, catalyze the chemical reactions that allow cells to function. � Storage, Myoglobin is an example of a storage protein. Myoglobin stores oxygen in muscles so that during exercise a ready supply of oxygen is available in the muscle tissue. � Hormones, some hormones are proteins, insulin is an example. Hormones serve as chemical messengers, carrying signals from one part of the body to another.

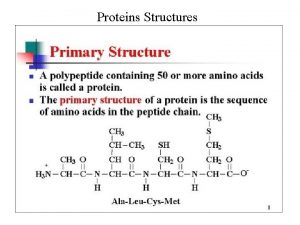
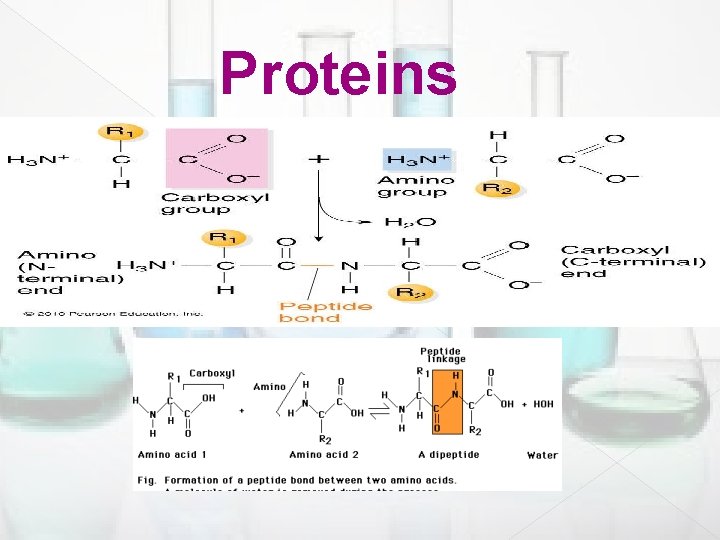
Peptide bond formation Polypeptides and proteins are held together by amide bonds between the amino end on one amino acid molecule, and the carboxylate end of another amino acid molecule, in a peptide , this amide bond is called a peptide bond. � The N terminus of a peptide/ protein is the end with its alpha amine not involved in a peptide bond. � The C terminus is the end with its carboxylic acid not involved in a peptide bond. �

� Primary structure � Secondary structure � Tertiary structure � Quaternary structure


Introduction � Properties of amino acids in proteins and peptides are determined by the R group but also by the charges of the titratable group. � Important to know which groups on peptides and proteins will be protonated at a certain p. H. � Protein molecules carry charges according to their amino acid sequence and the aqueous solvent PH they’re dissolved in.

Protein solubility There are many factors that contribute to protein solubility. � The most important determinant its electrostatic charge. � The solubility of proteins in aqueous buffers depends on the distribution of hydrophilic and hydrophobic amino acid residues on the protein’s surface. Proteins that have high hydrophobic amino acid content on the surface have low solubility in an aqueous solvent. �

Hydrophilic amino acid like: (Arginine, Asparagine, Aspartate, Glutamine, Glutamate, Histidine, Lysine, Serine and Threonine) � hydrophobic amino acid are (Valine, Tyrosine, Tryptophan, Proline, Phenylalanine, Methionine, Leucine, Isoleucine, Cysteine and Alanine ) � Charged and polar surface residues interact with ionic groups in the solvent and increase solubility. � The net charge of a protein molecule is the arithmetic average of all charges. At a certain solvent PH the protein net charge will be zero this is called the � isoelectirc point.

At a solution PH that is above the PI the surface of protein is predominantly negatively charged and therefore like charged molecules will exhibit repulsive forces. � Likewise the surface of the protein is predominantly positively charged at a solution PH that is below the PI, and repulsion between proteins occurs, so protein will be soluble at this PH. � However, at the PI the negative and positive charges are eliminated, repulsive electrostatic forces are reduced and the dispersive forces will cause aggregation and precipitation. � The PI of most proteins ranges between the PH 4 to 6. �

Since the solubility of protein such as casein is not affected by heat because it does not contain disulphide bonds and lack the tertiary structure. � The solubility of casein depends greatly on the PH of the medium. � The intermediate PH at which a protein molecule has a charge of zero is called, the isoelectric point of that protein. � At this point the solubility of protein is minimum, but increases with increasing acidity or alkalinity �

� The phenomenon of precipitation or coagulation of milk protein casein at low PH as milk becomes spoiled is one of the common examples of protein isolation due to changes in the PH.

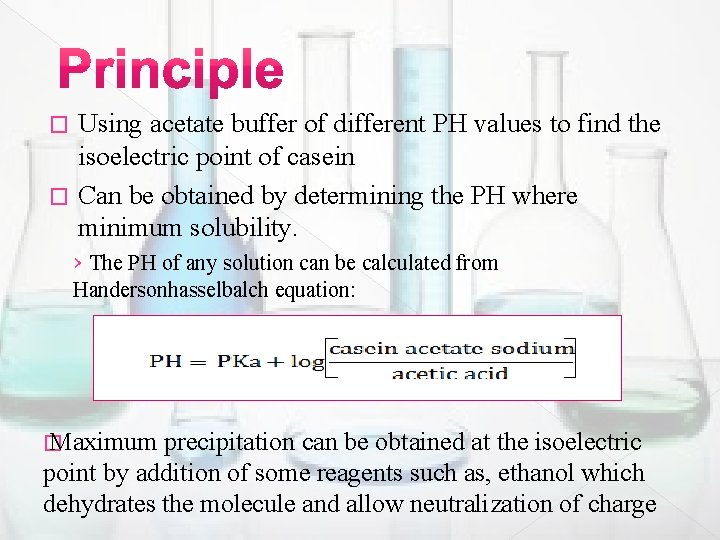
Using acetate buffer of different PH values to find the isoelectric point of casein � Can be obtained by determining the PH where minimum solubility. � › The PH of any solution can be calculated from Handersonhasselbalch equation: � Maximum precipitation can be obtained at the isoelectric point by addition of some reagents such as, ethanol which dehydrates the molecule and allow neutralization of charge

Proteins tend to aggregate and precipitate at their p. I because there is no electrostatic repulsion keeping them apart. � Proteins have different p. I because of their different amino acid sequences (i. e. , relative numbers of anionic and cationic groups), and thus they can be separated by adjusting the p. H of a solution. When the p. H is adjusted to the p. I of a particular protein it precipitates leaving the other proteins in solution. �

Experiment

Materials volumetric flask Protein(casein) 1 N Na. OH 1 N Acetic Acid 9 Test tubes H 2 O

Into a 50 ml volumetric flask add 20 ml of water. 2. Add 0. 25 g of pure casein, followed by the addition of 5 ml of 1 N Na. OH solution. 3. Once casein is dissolved, add 5 ml of 1 N acetic acid solution, then dilute with H 2 O to 50 ml and mix well. The resulted solution is a 0. 1 N casein acetate sodium. 1.

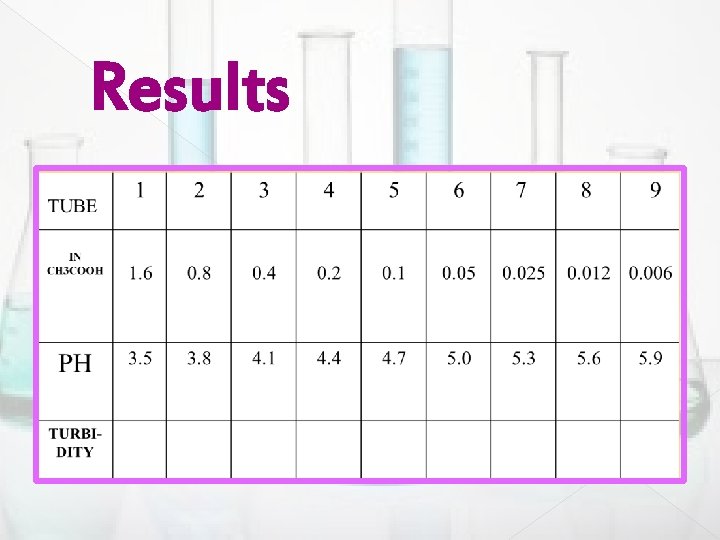
4. 5. 6. 7. 8. Setup a series of 9 test tubes to identify the best tube that have the most precipitate PI. In the first test tube put 3. 2 ml 1 N CH 3 COOH, and 6. 8 ml H 2 O and mix thoroughly. In each of the other test tubes (2 -9) put 5 ml H 2 Od. From the test tube 1 transfer 5 ml to the test tube 2, and mix thoroughly. Repeat step 7 for the rest of test tubes (3 - 9).

9. Now to each test tube (1 -9) add 1 ml of the casein acetate sodium solution, and shake the test tubes immediately. 10. Let the samples stand for 30 min, and note the turbidity in the 9 test tubes. 11. Use (+) and (– )signs to describe the turbidity in the different test tubes. 12. You should observe the most precipitation in the test tube which has the p. H around 4. 7 (close to the isoelectric point of casein).

Volumetric flasks are used to prepare various kinds of solutions the neck is narrow so that slight errors in reading the meniscus results in relatively small volumetric differences minimizes volumetric differences or errors. � A volumetric flask is used to make up a solution of fixed volume very accurately. �

Results

� Results Sheet � Results: � PKa = 4. 5 � Use the following to indicate the precipitate: � - no precipitate � + few ppt � + + Moderate ppt � + + + maximum ppt

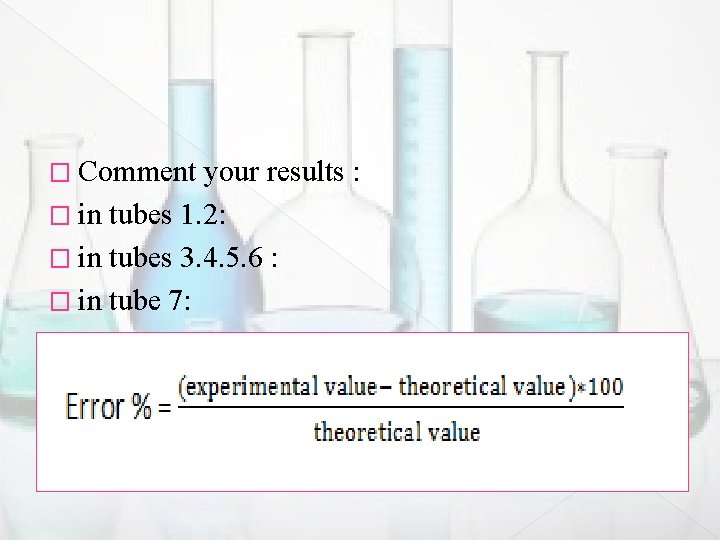
� Comment your results : � in tubes 1. 2: � in tubes 3. 4. 5. 6 : � in tube 7:

Thank
 Isoelectric point precipitation
Isoelectric point precipitation Non essential amino acids mnemonics
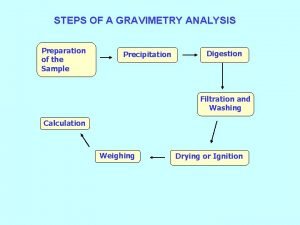
Non essential amino acids mnemonics Steps of gravimetric analysis
Steps of gravimetric analysis Co precipitation and post precipitation
Co precipitation and post precipitation Precipitation of proteins by strong mineral acids
Precipitation of proteins by strong mineral acids Isoelectric series
Isoelectric series Isoelectric focusing
Isoelectric focusing Isoelectric line
Isoelectric line Cardiac rhythms and interventions
Cardiac rhythms and interventions Which is the most common type of precipitation?
Which is the most common type of precipitation? Prep 1
Prep 1 Laser precipitation monitor
Laser precipitation monitor On which station model would the present weather symbol
On which station model would the present weather symbol Precipitation curve immunology
Precipitation curve immunology Causes of precipitation
Causes of precipitation Temperature of taiga
Temperature of taiga What clouds have the greatest turbulence?
What clouds have the greatest turbulence? Deciduous forest precipitation
Deciduous forest precipitation Pictures of temperate deciduous forest
Pictures of temperate deciduous forest Precipitation reaction word equation
Precipitation reaction word equation Long periods of unusually low precipitation are called
Long periods of unusually low precipitation are called Kcl precipitate
Kcl precipitate In greek language hudor means *
In greek language hudor means * A picture of precipitation
A picture of precipitation Chaparral precipitation
Chaparral precipitation Pictures of precipitation
Pictures of precipitation Wpc probabilistic winter precipitation guidance
Wpc probabilistic winter precipitation guidance