Proteins Structure and Function PROTEINS Proteins are essential
















- Slides: 24

Proteins Structure and Function

PROTEINS Proteins are essential to the structures and activities of life • A protein is a polymer constructed from amino acid monomers • The structure of the protein determines its function. • Most versatile macromolecules in our cells

Protein Functions The seven major functions of protein are 1) Structural: hair, cell cytoskeleton 2) Contractile: producers of movement in muscle and other cells 3) Storage: sources of amino acids, such as egg white 4) Defense: antibodies, membrane proteins 5) Transport: membrane proteins on cell membrane, carrier molecules such as hemoglobin (carry O 2 and CO 2) 6) Signaling: hormones, membrane proteins 7) Enzymes: regulators of the speed biochemical reactions

Amino acids • Monomeric subunit of polypeptides (proteins) • Have amino group and carboxyl group • Protein diversity is based on different arrangements of a common set of 20 amino acid monomers • differently

• 20 natural amino acids – Each has different R group (20 different “R” groups) – Differences in R group makes amino acids react • The structure of the R group determines the specific properties of each amino acid Properties defined by R-group include: – Polarity (non-polar, polar or charged) – Acidity (acidic, basic, neutral) – Soluble (hydrophillic, hydrophobic)

Essential Amino Acids • Humans can not synthesize them; they are dietary requirements (there are 8)


• proteins = peptides (short proteins) or polypeptides (long proteins) • Cells link amino acids together by dehydration synthesis • The bonds between amino acid monomers are called peptide bonds • Dipeptides are two amino acids long; polypeptides are from several to more than a thousand amino acids long Carboxyl group Amino acid Amino group Amino acid Peptide bond Dehydration reaction Dipeptide

Synthesis of Polypeptides • Polypeptide is synthesized by dehydration reaction • Chain grows from amino terminus to carboxy terminus • Chain has a repetitive backbone with variable side groups • R groups frequently interact with others

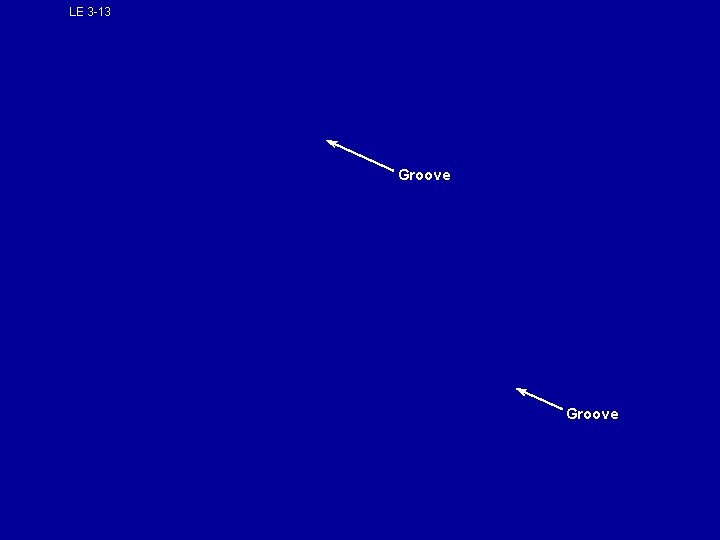
A protein's specific shape determines its function • • The order of amino acids determines which R groups will be able to interact and allow the protein to fold and coil in a unique way. This unique folding gives each protein their specific function. A protein consists of one or more polypeptide chains spontaneously folded into a unique shape The folding of a polypeptide creates grooves that enable other molecules to bind to it

LE 3 -13 Groove

A protein's shape depends on four levels of structure • • Primary structure Secondary structure Tertiary structure Quaternary structure

Primary Structure • Sequence of amino acids within a single polypeptide • amino acids linked by the peptide bond • dictates the final 3 dimensional shape of a protein • Are often similar among proteins of similar function • Usually written from amino terminus to carboxy terminus

Secondary Structure • Localized within regions of polypeptide • Stabilized by hydrogen bonding between the polar R groups • a helix-stabilized by frequent polar groups • b-pleated sheets are formed by consecutive polar groups on two regions of polypeptide repetitive or regular folding patterns

Tertiary Structure • Large folding events that are stabilized by interactions between amino acids irregular folding • 3 Dimension shape emerges Due to R group Interactions -Hydrogen bonds form between polar groups – Hydrophobic interactions • Nonpolar regions generally internalize in structure – Disulfide bridge • Very stable bond formed between two distant cysteine residues – Ionic interactions • Strong bond between oppositely charged side groups


Quaternary Structure • found in proteins made-up of more than one polypeptide chain to form one large globular protein • Interactions are maintained between polypeptide chains by bonds similar to tertiary structure • H-bonds, ionic interactions, hydrophobic interactions and disulphide bridges

Protein Structure Revisited

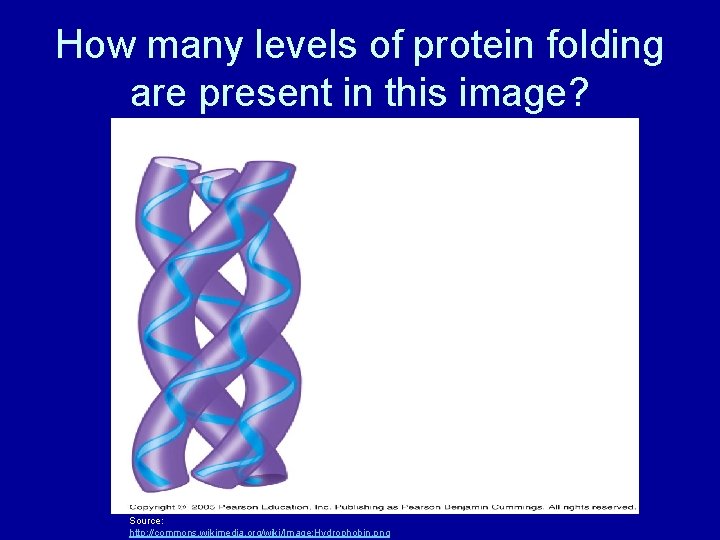
How many levels of protein folding are present in this image? Source: http: //commons. wikimedia. org/wiki/Image: Hydrophobin. png

• Collagen is an example of a protein with a quaternary structure – Three subunits wound into a helix – Structure provides great strength to long fibers


Factors Affecting Folding • Folding is influenced by environmental conditions – temperature – p. H – ionic concentration • Some proteins have more than one folding pattern (conformation) depending on the environment • Many proteins undergo a conformational change when a substrate binds. • Conformational changes are usually reversible.

Denaturation • Significant deviation from the “native state” of a protein causes denaturation (unfolding) • Denatured proteins tend to aggregate (clump together) due to the exposure of hydrophobic R groups • Denatured proteins may refold if the conditions are appropriate • Some proteins resist denaturation due to the presence of special enzymes called chapperones • Chapperones promote or stabilise a protein’s folding

Proteins when heated can unfold or "Denature". This loss of three dimensional shape will usually be accompanied by a loss of the proteins function. If the denatured protein is allowed to cool it will usually refold back into it’s original conformation.