Proteins Qualitative Tests for Proteins n Proteins are





- Slides: 21

+ Proteins Qualitative Tests for Proteins

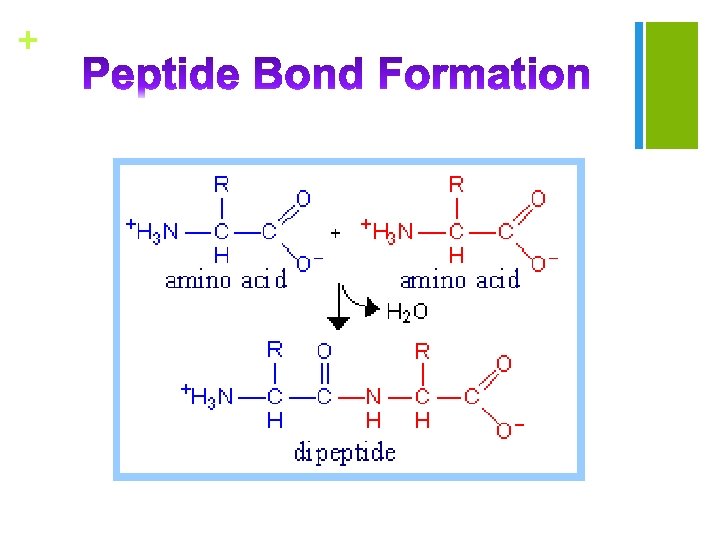
+ n Proteins are derived from the Greek protas meaning "of primary importance”) n Complex, n high-molecular-weight molecules Biochemical molecules that consists of amino acids joined by peptide bonds n Proteins can be hydrolyzed by acids, bases or specific enzymes. n Proteins are probably the most important class of biochemical molecules


+ n Denaturation is the disruption of secondary, tertiary and quaternary structure of proteins leading to loss of their biological activity. n Proteins denature when they lose their threedimensional structure. n Proteins may be denatured at the secondary, tertiary and quaternary structural levels, but not at the primary structural level.

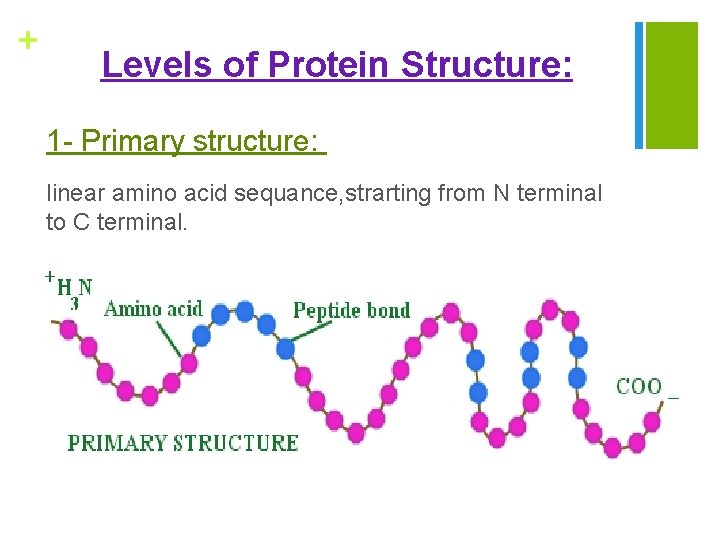
+ Levels of Protein Structure: 1 - Primary structure: linear amino acid sequance, strarting from N terminal to C terminal.

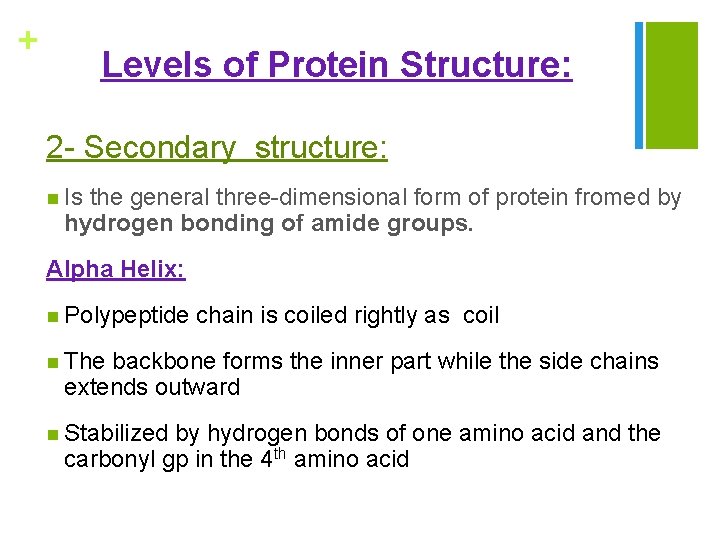
+ Levels of Protein Structure: 2 - Secondary structure: n Is the general three-dimensional form of protein fromed by hydrogen bonding of amide groups. Alpha Helix: n Polypeptide chain is coiled rightly as coil n The backbone forms the inner part while the side chains extends outward n Stabilized by hydrogen bonds of one amino acid and the carbonyl gp in the 4 th amino acid

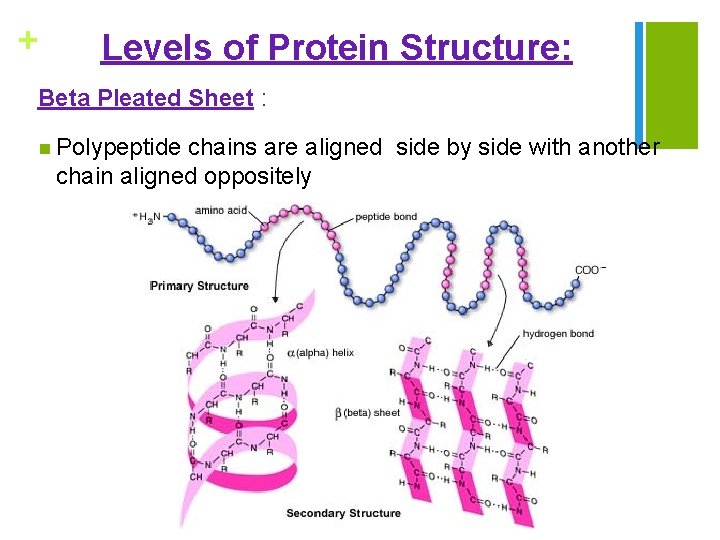
+ Levels of Protein Structure: Beta Pleated Sheet : n Polypeptide chains are aligned side by side with another chain aligned oppositely

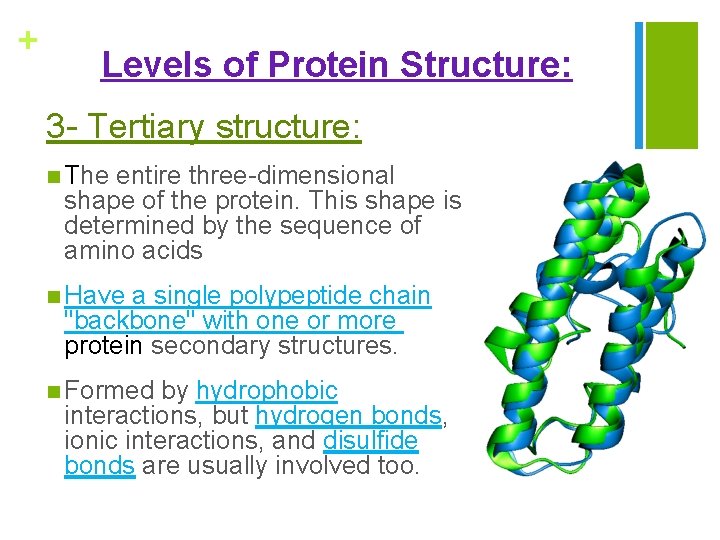
+ Levels of Protein Structure: 3 - Tertiary structure: n The entire three-dimensional shape of the protein. This shape is determined by the sequence of amino acids n Have a single polypeptide chain "backbone" with one or more protein secondary structures. n Formed by hydrophobic interactions, but hydrogen bonds, ionic interactions, and disulfide bonds are usually involved too.

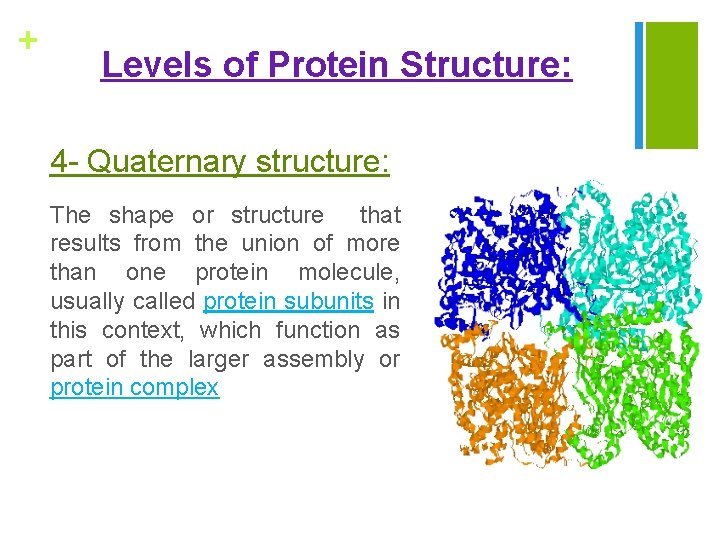
+ Levels of Protein Structure: 4 - Quaternary structure: The shape or structure that results from the union of more than one protein molecule, usually called protein subunits in this context, which function as part of the larger assembly or protein complex

+ Causes of denaturation Physical factors such as heating Chemical factors such as strong acid or base

+ Characteristics of denatured proteins are : 1 -Loss of function: Most biological proteins lose their biological function when denatured, for example, enzymes lose their catalytic activity 2 - They become less soluble. As a result, they are easily precipitated. 3 - Reversibility and irreversibility: In many proteins (unlike egg whites), denaturation is reversible (the proteins can regain their native state when the denaturing influence is removed).

+ Qualitative Tests for Proteins

+ 1 - Biuret Test q It is the general test for all proteins. q Biuret reagent is dilute Cu. SO 4 in strong alkaline medium. q Alkaline Cu. SO 4 reacts with all compounds containing 2 or more peptide bonds to give a blue-violet color. Method: 1 ml of biuret reagent + 1 ml of protein ……mix well>>>> blue-violet color.

+ 2 - Denaturation by heat and extreme p. H: n Extreme heating and p. H (conc. acids) denature proteins leading to precipitation of proteins. Method: n 3 ml Protein >>>>>>BWB-10 min >>>>>> ppt of protein. Protein >>>>>> drops conc. HCL >>>>>> ppt of protein.

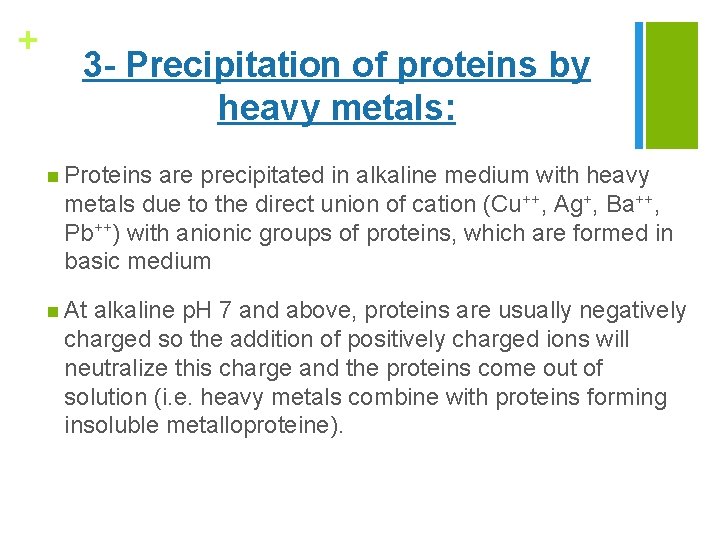
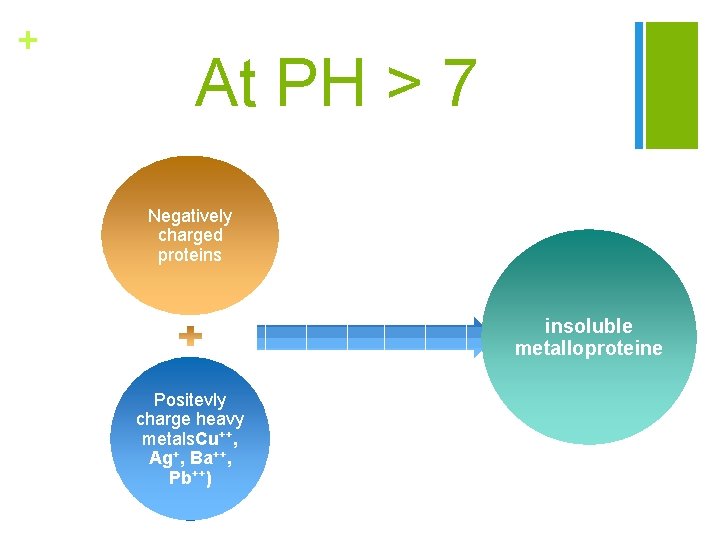
+ 3 - Precipitation of proteins by heavy metals: n Proteins are precipitated in alkaline medium with heavy metals due to the direct union of cation (Cu++, Ag+, Ba++, Pb++) with anionic groups of proteins, which are formed in basic medium n At alkaline p. H 7 and above, proteins are usually negatively charged so the addition of positively charged ions will neutralize this charge and the proteins come out of solution (i. e. heavy metals combine with proteins forming insoluble metalloproteine).

+ At PH > 7 Negatively charged proteins insoluble metalloproteine Positevly charge heavy metals. Cu++, Ag+, Ba++, Pb++)

+ Method: n Few drops of heavy metals + 2 ml protein + few drops 10% Na. OH>>>>ppt

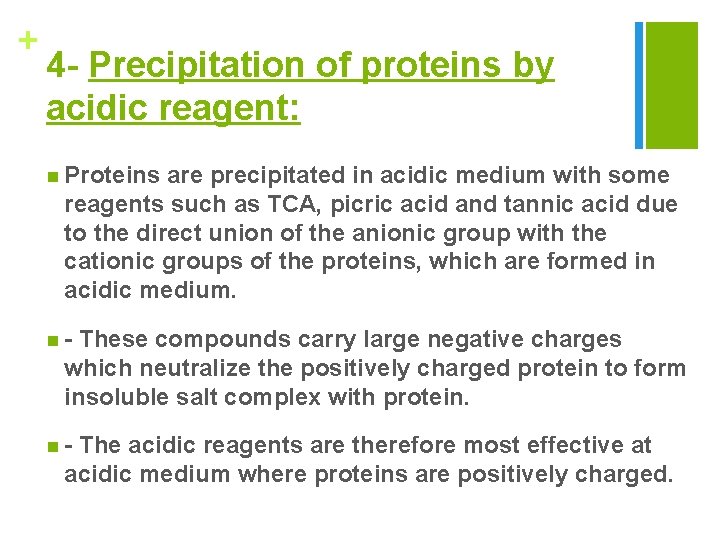
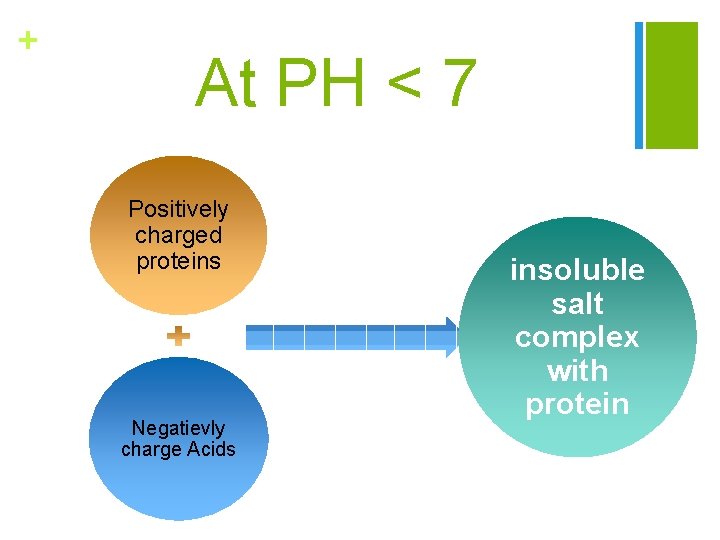
+ 4 - Precipitation of proteins by acidic reagent: n Proteins are precipitated in acidic medium with some reagents such as TCA, picric acid and tannic acid due to the direct union of the anionic group with the cationic groups of the proteins, which are formed in acidic medium. n- These compounds carry large negative charges which neutralize the positively charged protein to form insoluble salt complex with protein. n- The acidic reagents are therefore most effective at acidic medium where proteins are positively charged.

+ At PH < 7 Positively charged proteins Negatievly charge Acids insoluble salt complex with protein

+ Method: n Few drops of acidic reagent + 2 ml protein >>>slowly add dilute Na. OH and observe the result as the p. H increase.

+ Practical n Perform gelatine the previous tests on Albumin, Peptone an