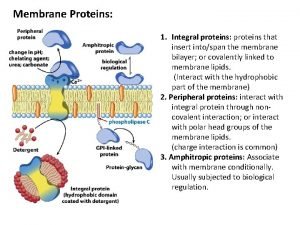
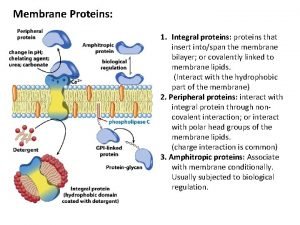
PROTEINS Proteins Proteins are made of long strings





























- Slides: 76

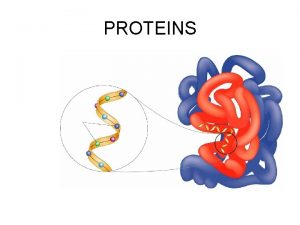
PROTEINS

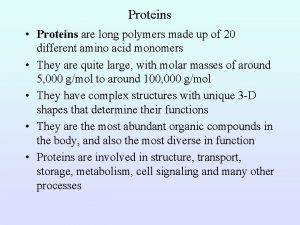
Proteins • Proteins are made of long strings of individual building blocks known as amino acids.

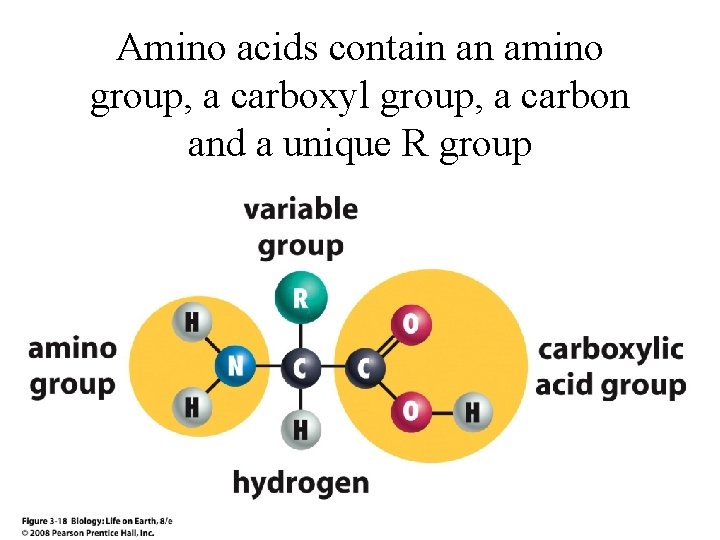
Amino acids contain an amino group, a carboxyl group, a carbon and a unique R group

3. 2. 2 • Identify amino acids. . . from diagrams showing their structure.

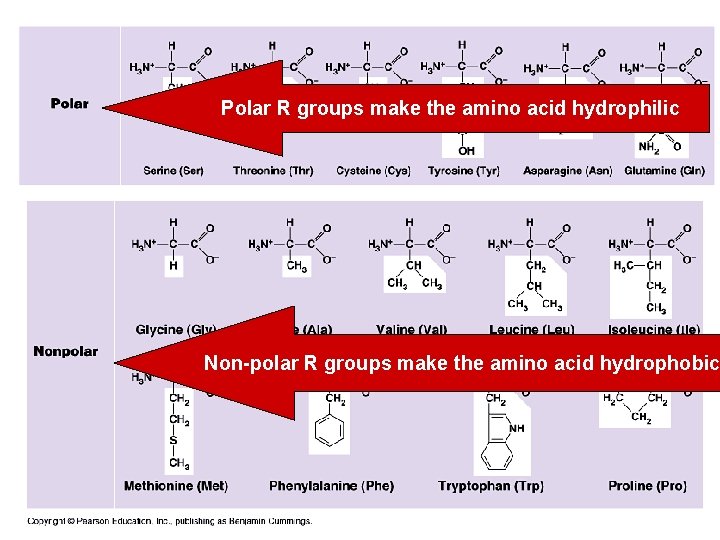
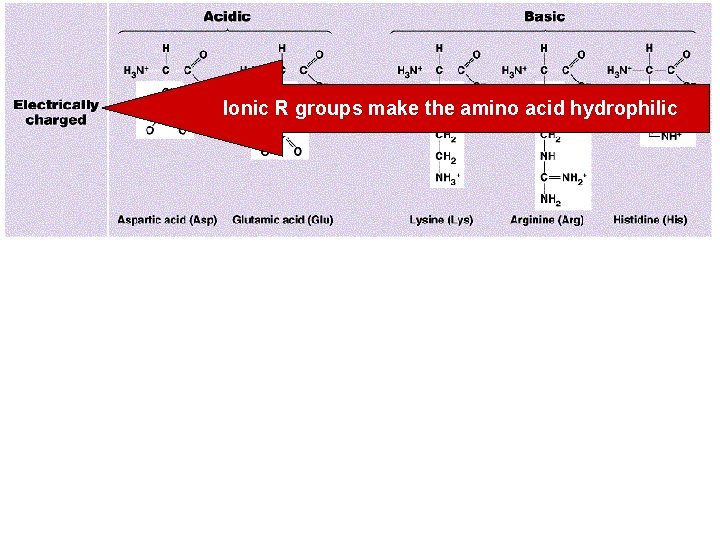
Polar R groups make the amino acid hydrophilic Non-polar R groups make the amino acid hydrophobic

Ionic R groups make the amino acid hydrophilic

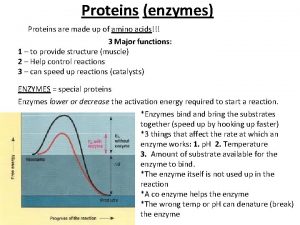
7. 5. 3 • Explain the significance of polar and non-polar amino acids.

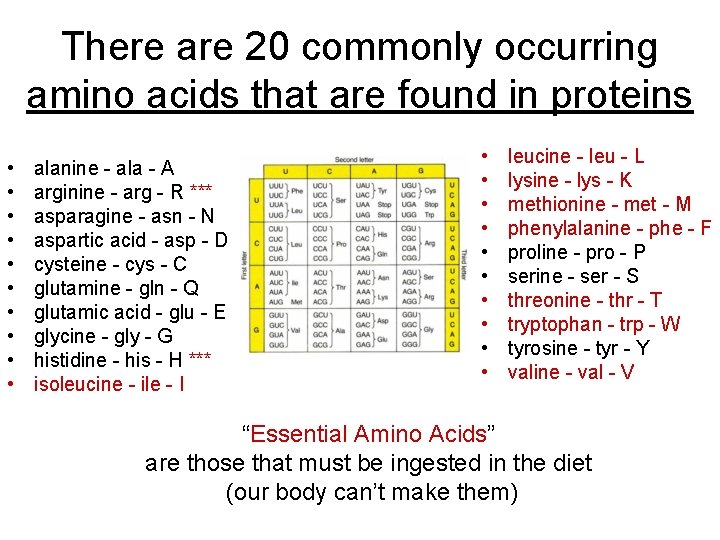
There are 20 commonly occurring amino acids that are found in proteins • • • alanine - ala - A arginine - arg - R *** asparagine - asn - N aspartic acid - asp - D cysteine - cys - C glutamine - gln - Q glutamic acid - glu - E glycine - gly - G histidine - his - H *** isoleucine - ile - I • • • leucine - leu - L lysine - lys - K methionine - met - M phenylalanine - phe - F proline - pro - P serine - ser - S threonine - thr - T tryptophan - trp - W tyrosine - tyr - Y valine - val - V “Essential Amino Acids” are those that must be ingested in the diet (our body can’t make them)

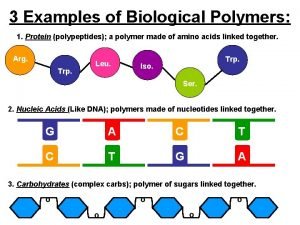
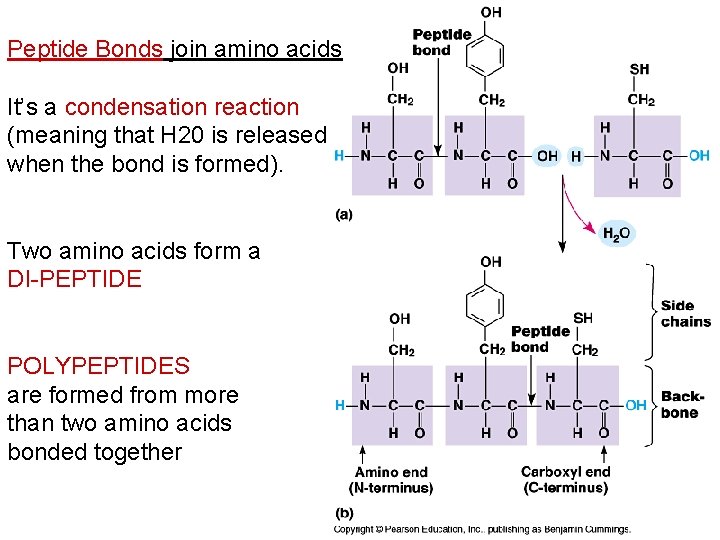
Peptide Bonds join amino acids It’s a condensation reaction (meaning that H 20 is released when the bond is formed). Two amino acids form a DI-PEPTIDE POLYPEPTIDES are formed from more than two amino acids bonded together


Outline the role of condensation and hydrolysis in the relationships between … amino acids and polypeptides. • 7. 4. 5 Draw and label a diagram showing the structure of a peptide bond between two amino acids. • 3. 2. 5

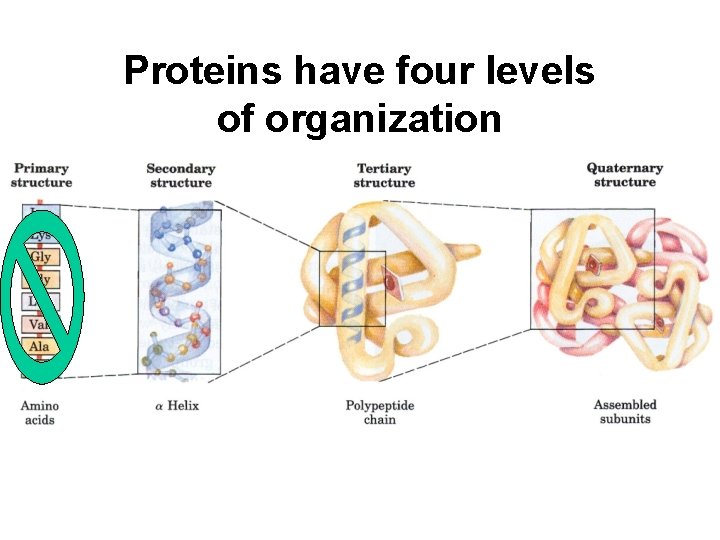
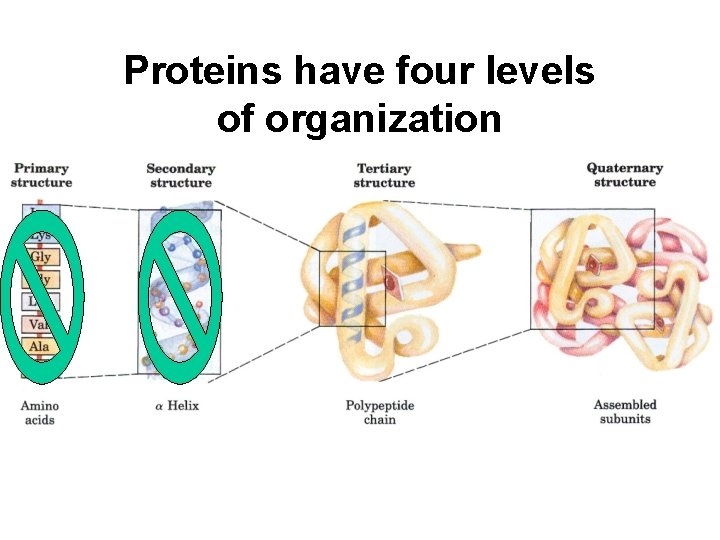
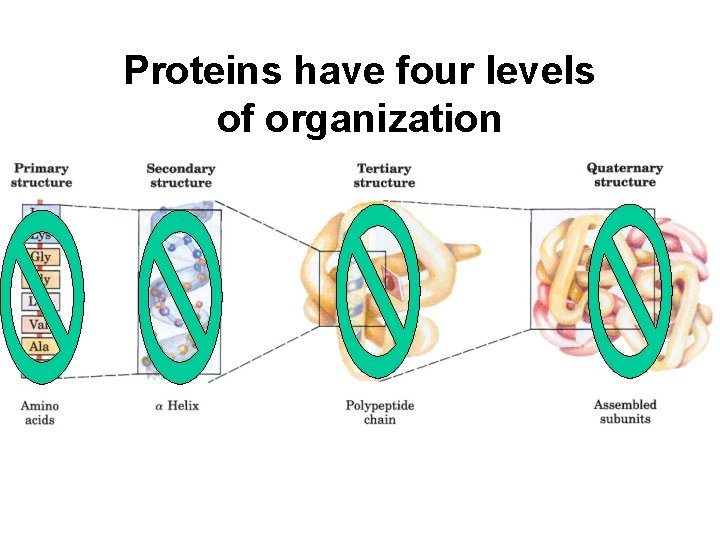
Proteins have four levels of organization

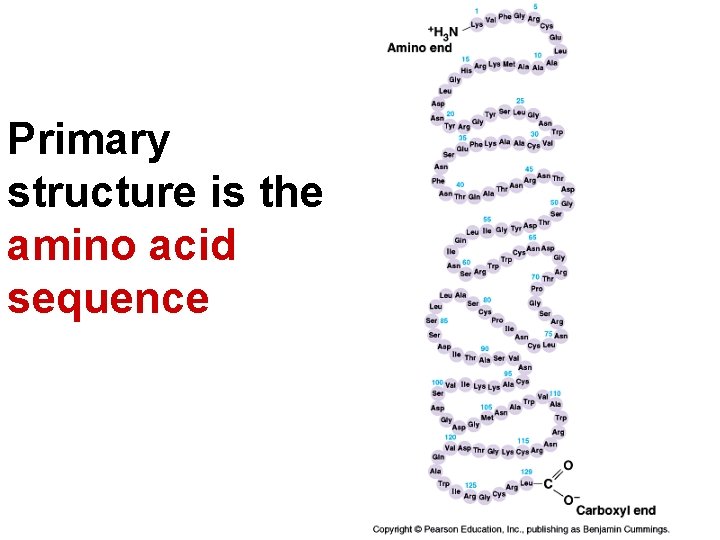
Primary structure is the amino acid sequence

The amino acid sequence is coded for by DNA and is unique for each kind of protein

The amino acid sequence determines how the polypeptide will fold into its 3 D shape

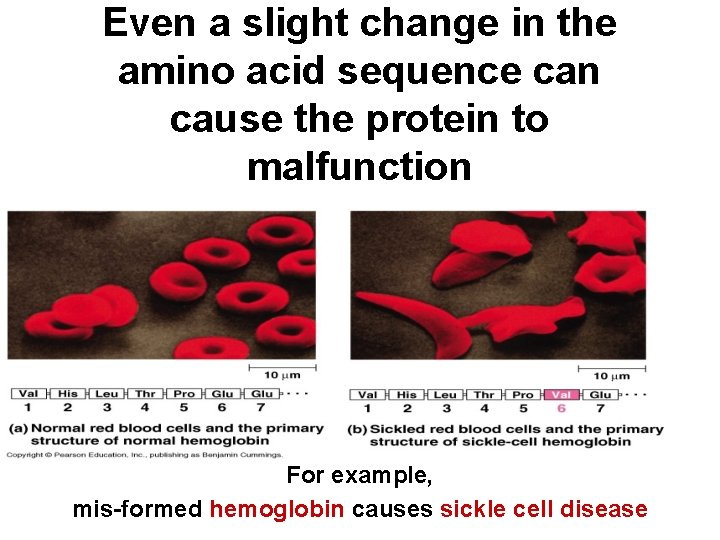
Even a slight change in the amino acid sequence can cause the protein to malfunction For example, mis-formed hemoglobin causes sickle cell disease


Proteins have four levels of organization

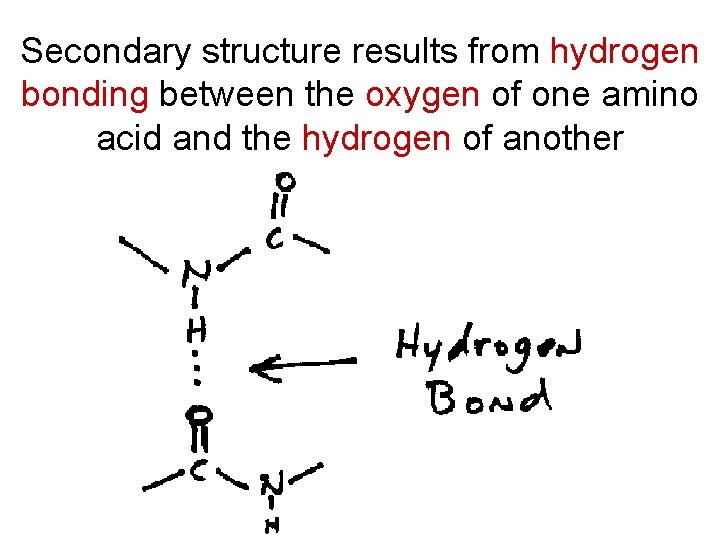
Secondary structure results from hydrogen bonding between the oxygen of one amino acid and the hydrogen of another


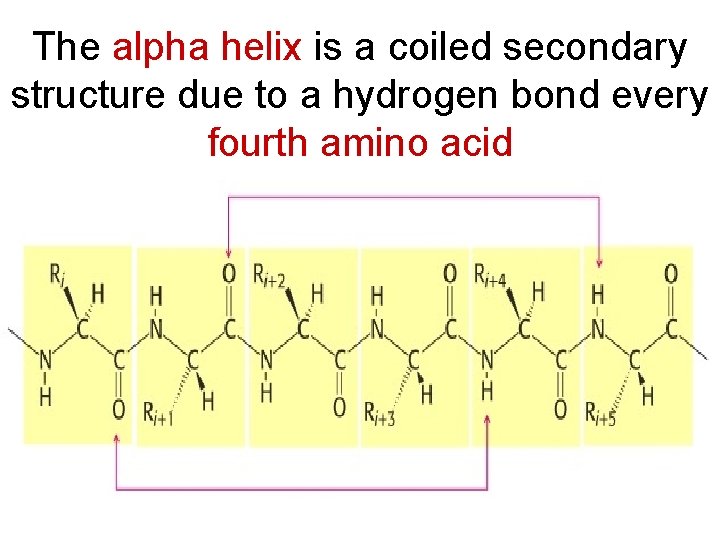
The alpha helix is a coiled secondary structure due to a hydrogen bond every fourth amino acid



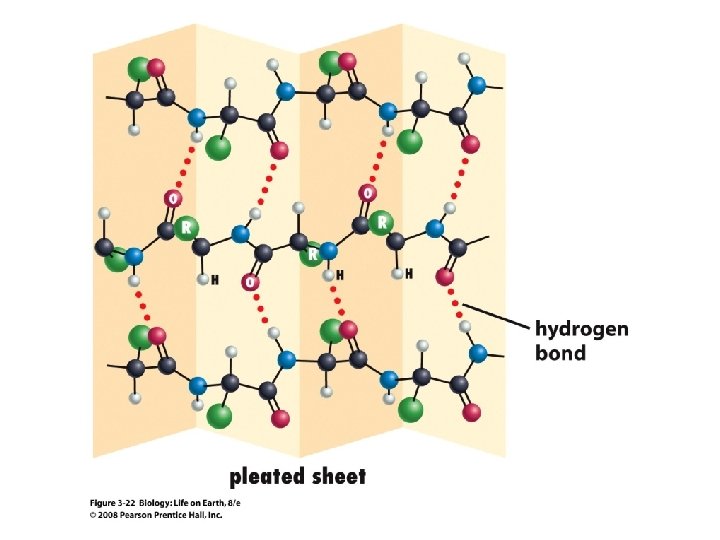
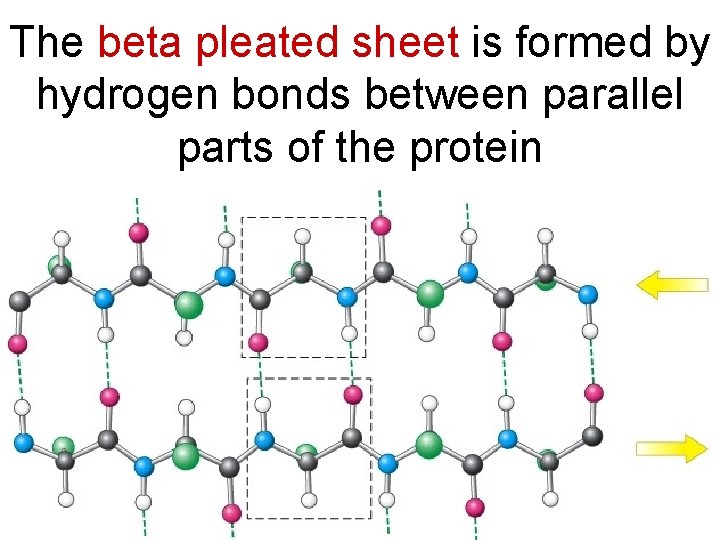
The beta pleated sheet is formed by hydrogen bonds between parallel parts of the protein


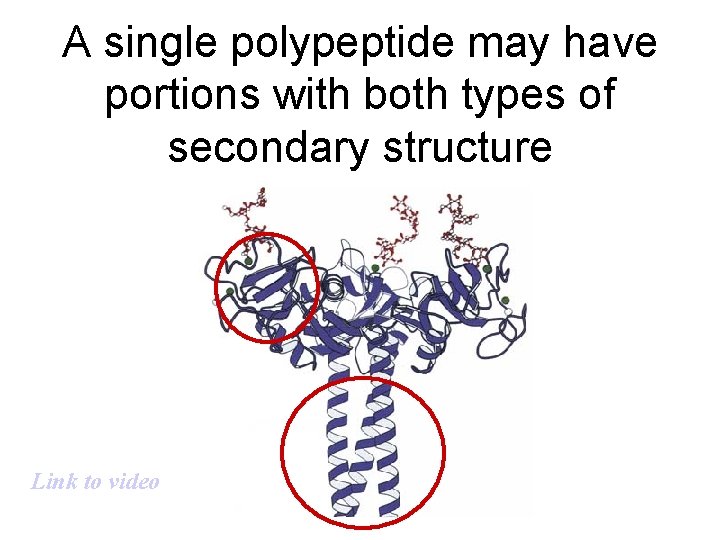
A single polypeptide may have portions with both types of secondary structure Link to video


Proteins have four levels of organization


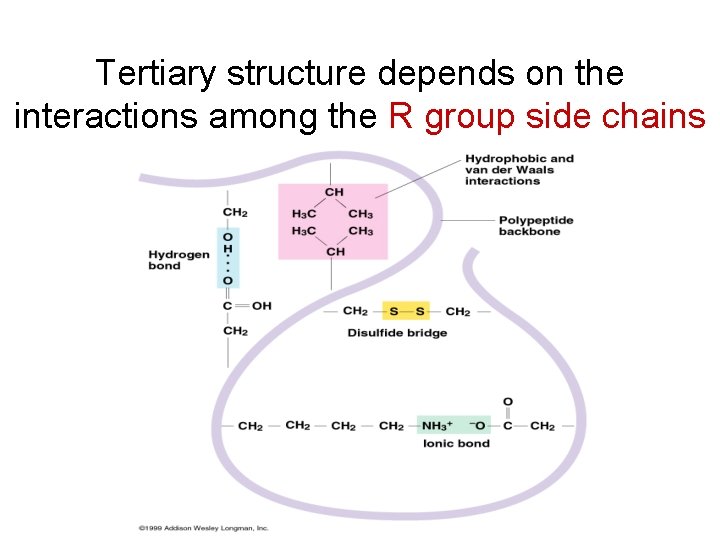
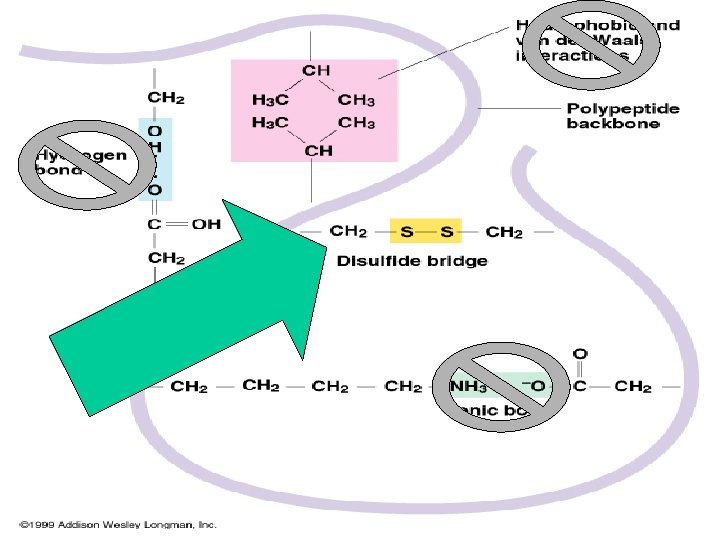
Tertiary structure depends on the interactions among the R group side chains


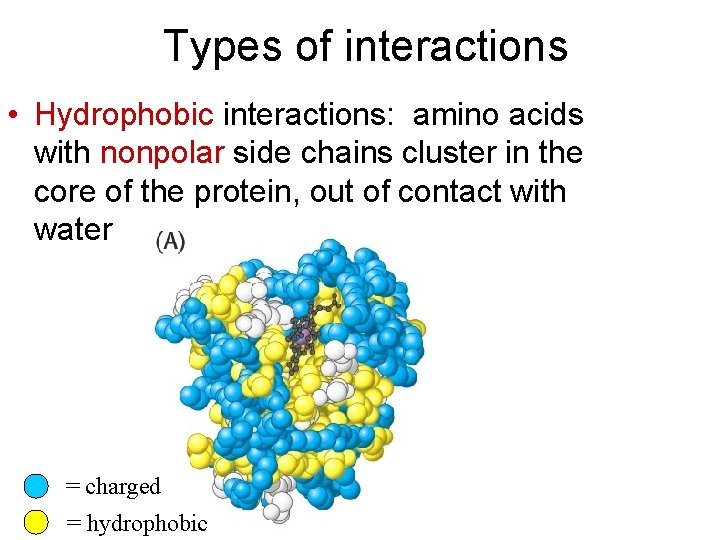
Types of interactions • Hydrophobic interactions: amino acids with nonpolar side chains cluster in the core of the protein, out of contact with water = charged = hydrophobic


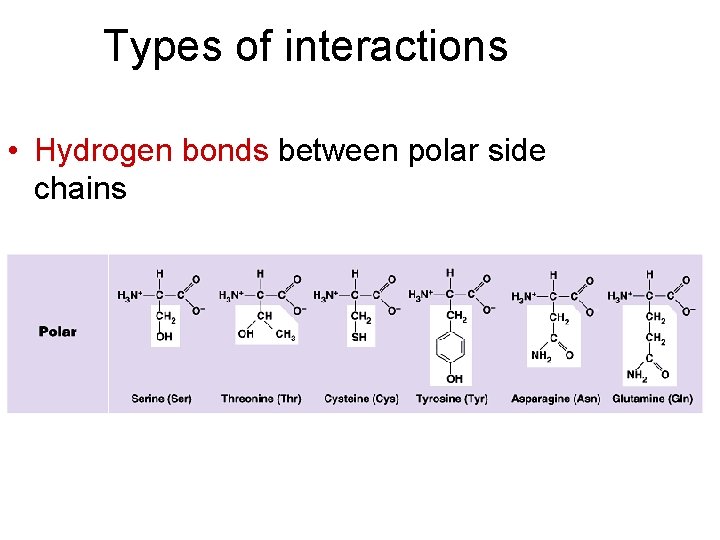
Types of interactions • Hydrogen bonds between polar side chains


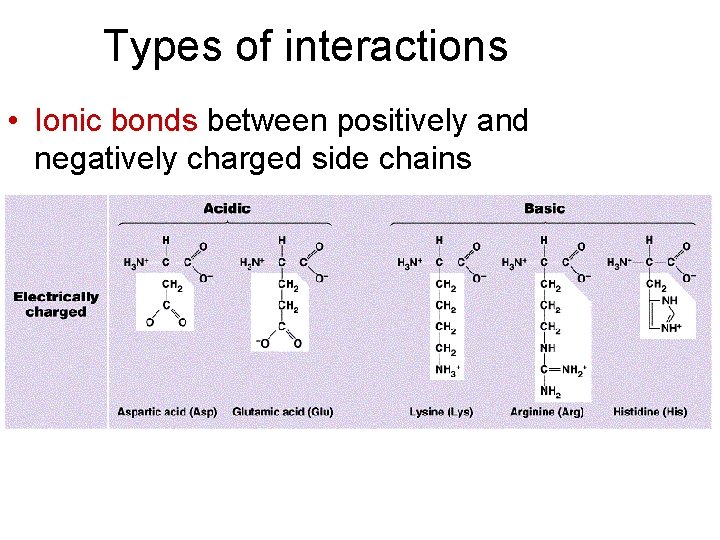
Types of interactions • Ionic bonds between positively and negatively charged side chains


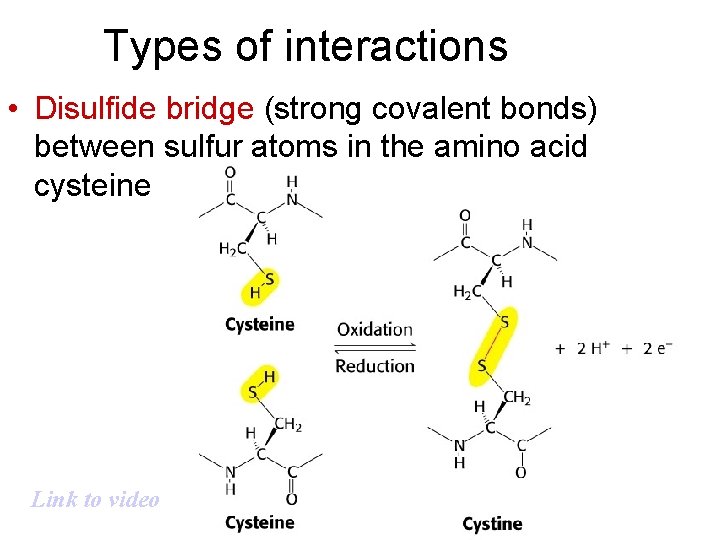
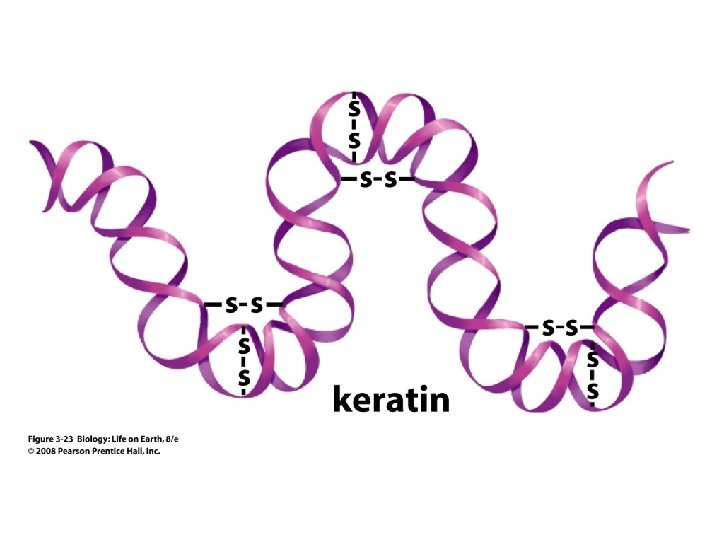
Types of interactions • Disulfide bridge (strong covalent bonds) between sulfur atoms in the amino acid cysteine Link to video



Proteins have four levels of organization

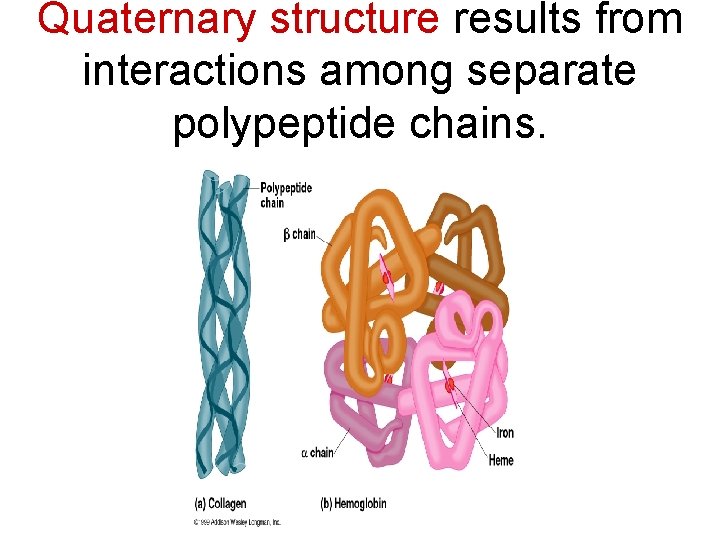
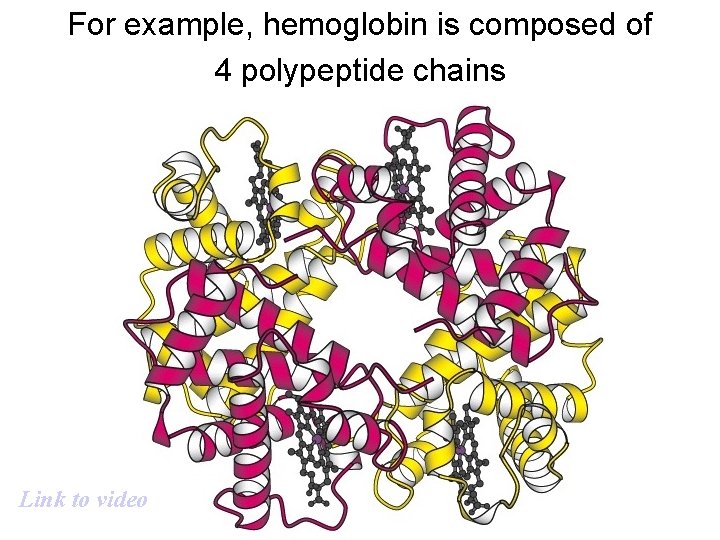
Quaternary structure results from interactions among separate polypeptide chains.

For example, hemoglobin is composed of 4 polypeptide chains Link to video


Proteins have four levels of organization

The folding of proteins is aided by other proteins, called chaperones • Act as temporary braces as proteins fold into their final conformation • Research into chaperones is a area of research in biology


7. 5. 1 • Explain the four levels of protein structure, indicating the significance of each level.

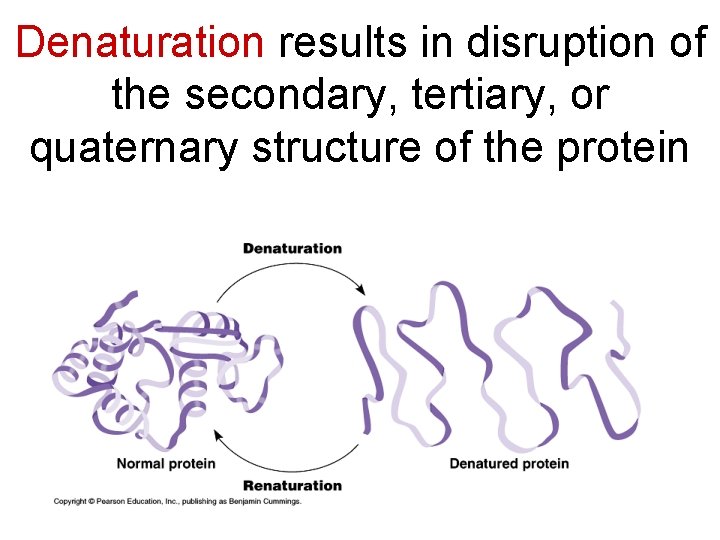
Denaturation results in disruption of the secondary, tertiary, or quaternary structure of the protein

Denaturation may be due to – changes in p. H, temperature or various chemicals

Protein function is lost during denaturation, which is often irreversible

3. 6. 4 • Define denaturation.

Folded proteins are placed into two general categories

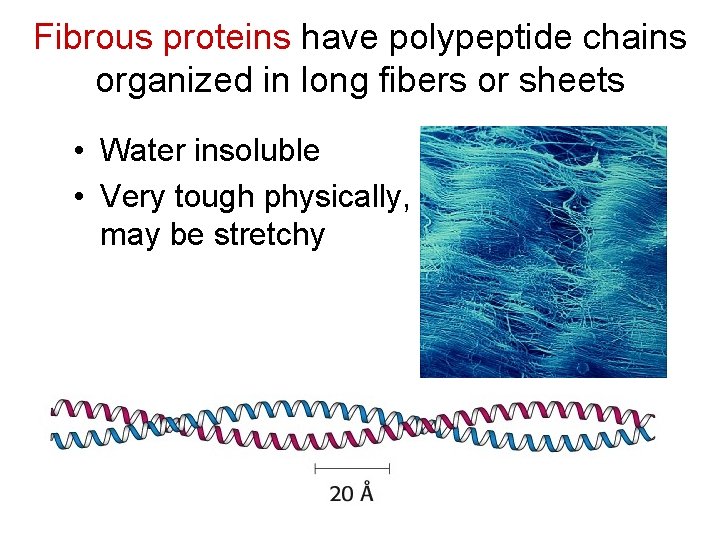
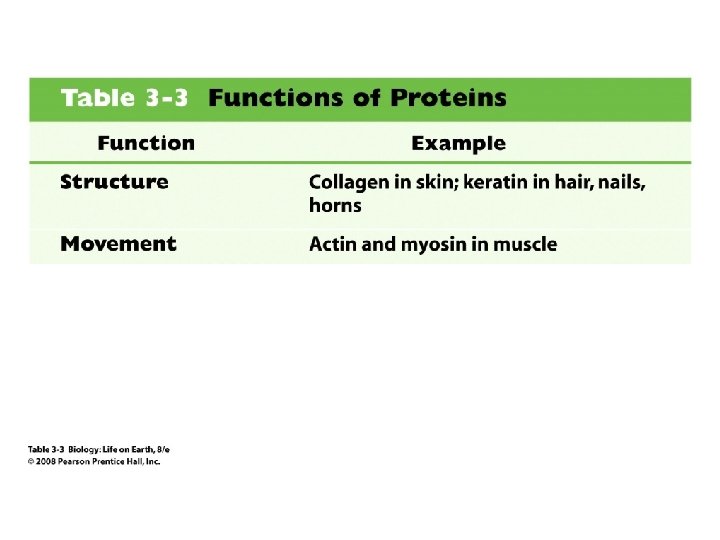
Fibrous proteins have polypeptide chains organized in long fibers or sheets • Water insoluble • Very tough physically, may be stretchy

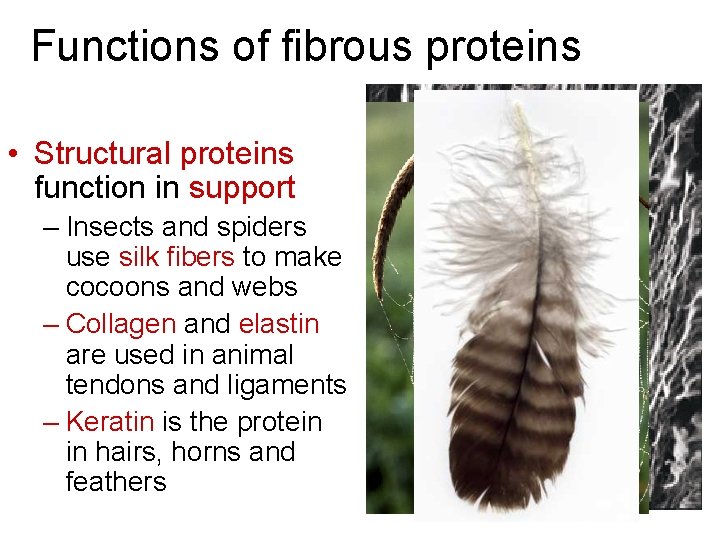
Functions of fibrous proteins • Structural proteins function in support – Insects and spiders use silk fibers to make cocoons and webs – Collagen and elastin are used in animal tendons and ligaments – Keratin is the protein in hairs, horns and feathers



Functions of fibrous proteins • Contractile proteins function in movement – Actin and myosin contract to create the cleavage furrow and to move muscles – Contractile proteins move cilia and flagella

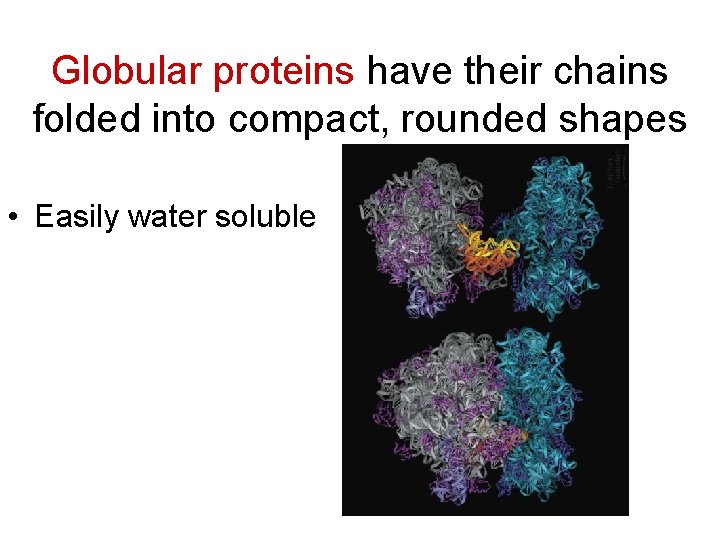
Globular proteins have their chains folded into compact, rounded shapes • Easily water soluble

Functions of globular proteins • Storage proteins function in the storage of amino acids – Ovalbumin is the protein in egg whites – Casein is the protein in milk, source of amino acids for baby mammals


Functions of globular proteins • Transport proteins function in the movement of other substances – Hemoglobin, the iron containing protein in blood, transport oxygen from lungs to other parts of the body (C 3032 H 4816 O 872 N 780 S 9 Fe 4) – Membrane transport proteins such as channels for potassium and water


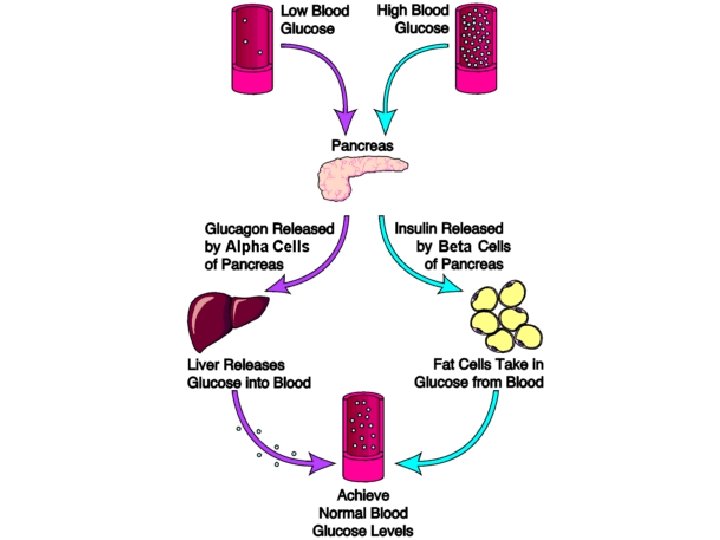
Functions of globular proteins • Hormone proteins function as cellular messenger molecules that help maintain homeostasis – Insulin: sends message “allow sugar into cells” (when blood glucose levels are high, cells will transport glucose into the cells for use or storage) – Glucagon: sends message “we need more sugar in the blood” (when blood glucose is too low, cells will release glucose)


Functions of globular proteins • Receptor proteins allow cells to respond to chemical stimuli – Growth factor receptors initiate the signal transduction pathway when a growth hormone attaches

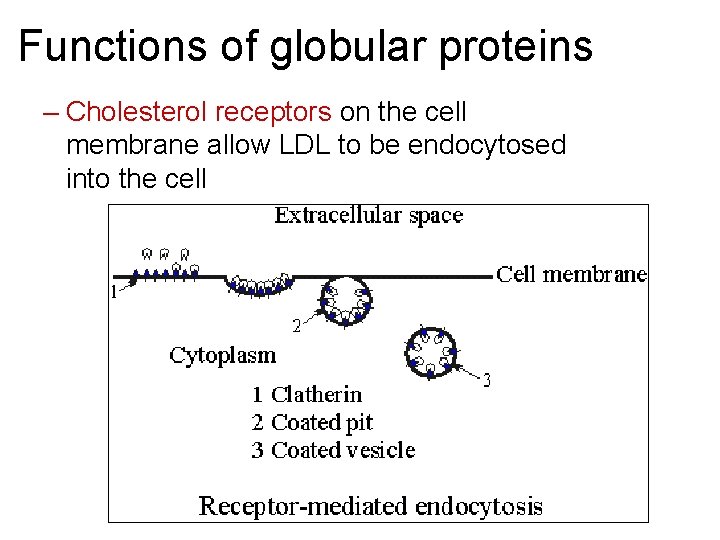
Functions of globular proteins – Cholesterol receptors on the cell membrane allow LDL to be endocytosed into the cell

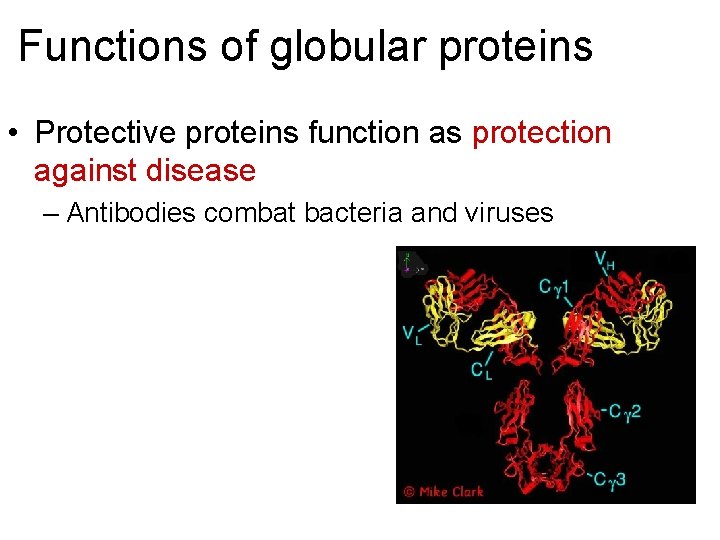
Functions of globular proteins • Protective proteins function as protection against disease – Antibodies combat bacteria and viruses

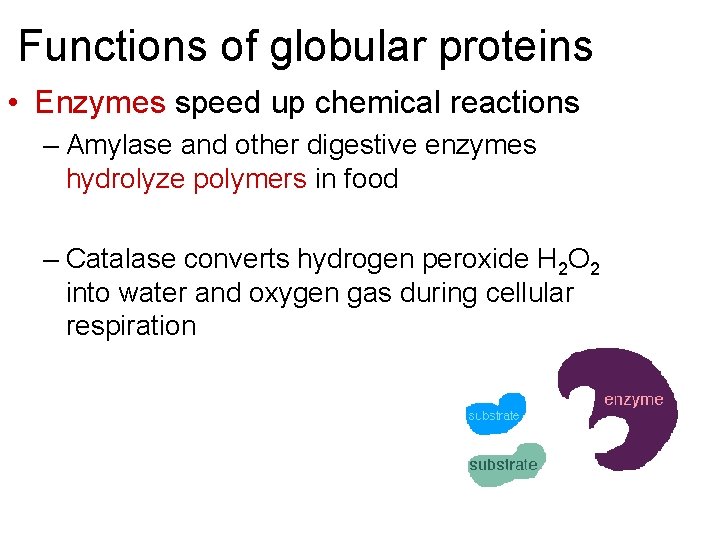
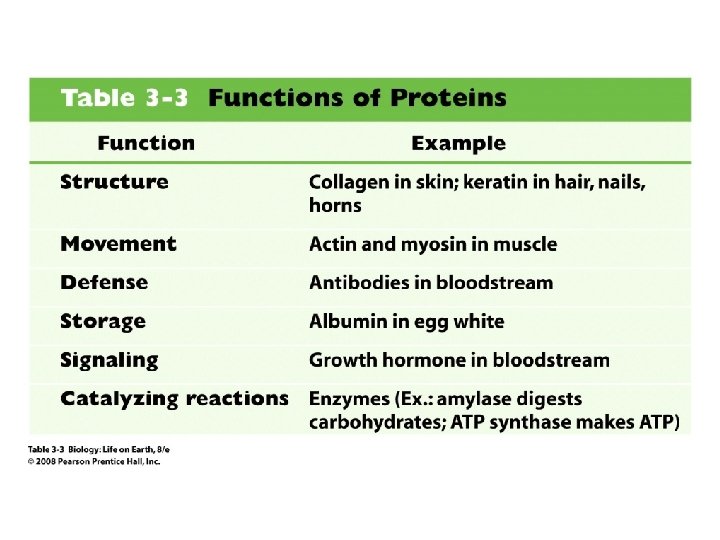
Functions of globular proteins • Enzymes speed up chemical reactions – Amylase and other digestive enzymes hydrolyze polymers in food – Catalase converts hydrogen peroxide H 2 O 2 into water and oxygen gas during cellular respiration







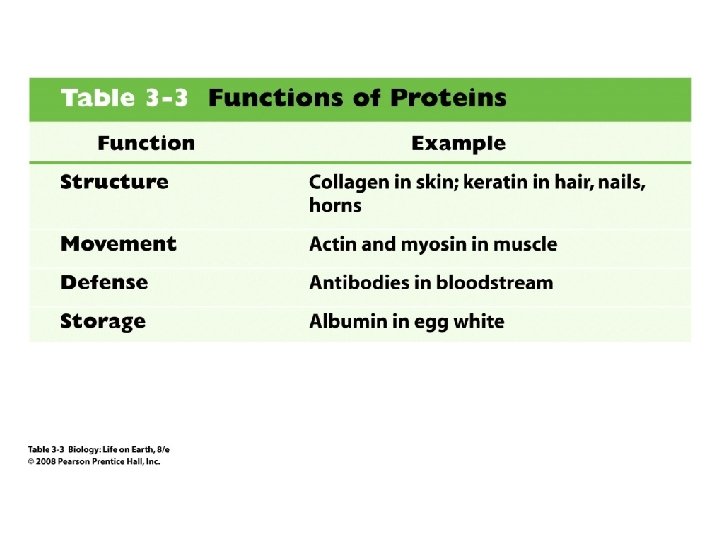
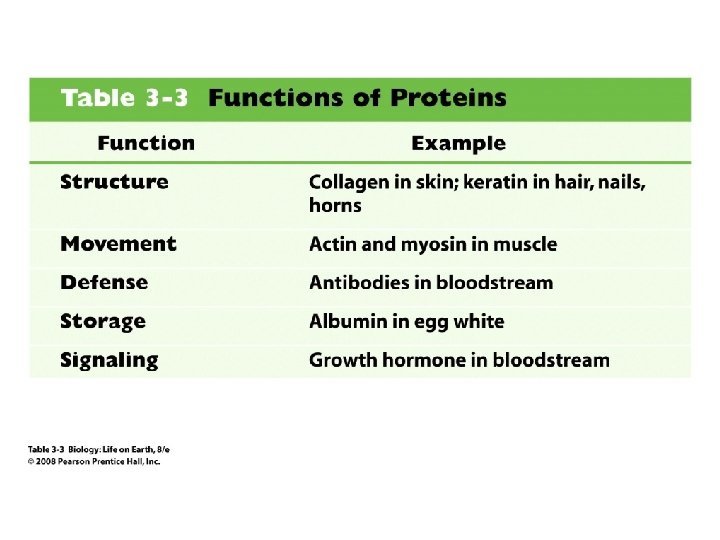
7. 5. 2 – Outline the difference between fibrous and globular proteins, with reference to two examples of each protein type. 7. 5. 4 – State four functions of proteins, giving a named example of each.
 Mikael ferm
Mikael ferm Serongagandi instrument description
Serongagandi instrument description Once upon a time a little man
Once upon a time a little man Short short short long long long short short short
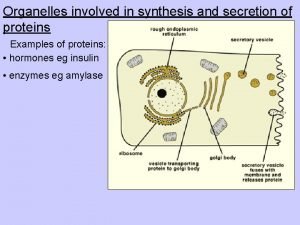
Short short short long long long short short short Which organelle is the site where proteins are made
Which organelle is the site where proteins are made Import java.util.string
Import java.util.string String in c
String in c Notation for sequences
Notation for sequences How many bit strings of length 10 contain
How many bit strings of length 10 contain Python compare strings
Python compare strings Declare a two dimensional array of strings named chessboard
Declare a two dimensional array of strings named chessboard Lflexion
Lflexion Cld instruction in 8086
Cld instruction in 8086 A type of cipher that uses multiple alphabetic strings.
A type of cipher that uses multiple alphabetic strings. Tension in elastic string
Tension in elastic string Jmp in excel
Jmp in excel Upx decompiler
Upx decompiler Pointers and strings
Pointers and strings String and other things
String and other things Things not strings
Things not strings Circular motion lab
Circular motion lab Flexible pattern
Flexible pattern Ida pro strings
Ida pro strings Funções de linguagem
Funções de linguagem Mason nagy
Mason nagy What are strings in c
What are strings in c Array of strings assembly
Array of strings assembly Three masses are connected by strings
Three masses are connected by strings Ottawa suzuki strings
Ottawa suzuki strings Rate of energy transfer by sinusoidal waves on strings
Rate of energy transfer by sinusoidal waves on strings Ida strings
Ida strings Vortex strings
Vortex strings Mrs sprockett's strange machine
Mrs sprockett's strange machine A long lasting paper like material made from reeds
A long lasting paper like material made from reeds Greek
Greek Tinikling costume and props
Tinikling costume and props Bọn em hai đứa cùng tên
Bọn em hai đứa cùng tên Once upon a time a long long time ago begins the story
Once upon a time a long long time ago begins the story Once upon a time a long long time ago begins the story
Once upon a time a long long time ago begins the story Long long ago people used to think that the earth was
Long long ago people used to think that the earth was Long long int c
Long long int c Cái gậy cạnh quả trứng gà
Cái gậy cạnh quả trứng gà Once upon a time there lived a king who was a fine man
Once upon a time there lived a king who was a fine man Does exocytosis require energy
Does exocytosis require energy Not all enzymes are proteins
Not all enzymes are proteins Food pyramid carbohydrates fats proteins
Food pyramid carbohydrates fats proteins Quantitative determination of proteins
Quantitative determination of proteins Ups trucks means which organelle
Ups trucks means which organelle Proteins are divided into two groups
Proteins are divided into two groups Storage proteins function
Storage proteins function Qualitative test for proteins
Qualitative test for proteins Proteins contain what elements
Proteins contain what elements Amphoteric proteins
Amphoteric proteins Introduction of protein
Introduction of protein Monomer of proteins
Monomer of proteins Svjetlana kalanj bognar
Svjetlana kalanj bognar Carrier vs channel proteins
Carrier vs channel proteins Structural role of proteins
Structural role of proteins Precipitation of proteins by strong mineral acids
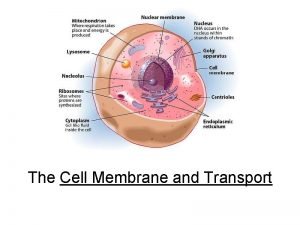
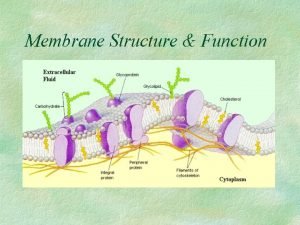
Precipitation of proteins by strong mineral acids Membrane synthesis
Membrane synthesis Amino acids are joined together in proteins by
Amino acids are joined together in proteins by Structural proteins function
Structural proteins function Monomers that make up proteins
Monomers that make up proteins Transdeamination of amino acids
Transdeamination of amino acids Polymer of protein example
Polymer of protein example Channel vs carrier proteins
Channel vs carrier proteins Neurotrasmiters
Neurotrasmiters Section 8-1 carbohydrates fats and proteins answer key
Section 8-1 carbohydrates fats and proteins answer key Three main types of rna
Three main types of rna Introduction of protein
Introduction of protein Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Non collagenous proteins of periodontium
Non collagenous proteins of periodontium Salting in
Salting in Elementary composition of proteins
Elementary composition of proteins Microtubules analogy
Microtubules analogy Cummings
Cummings L
L