How molecules are formed generally two other molecules




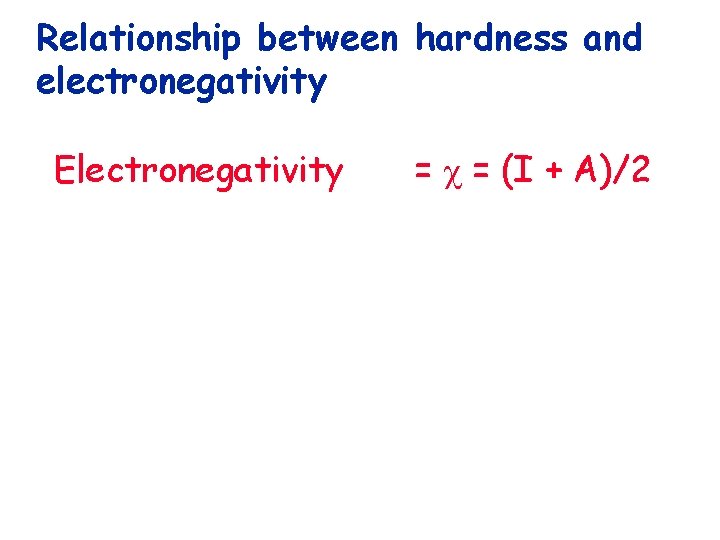



- Slides: 24

How molecules are formed: generally two other molecules react

How molecules are formed: generally two other molecules react What factors are involved?

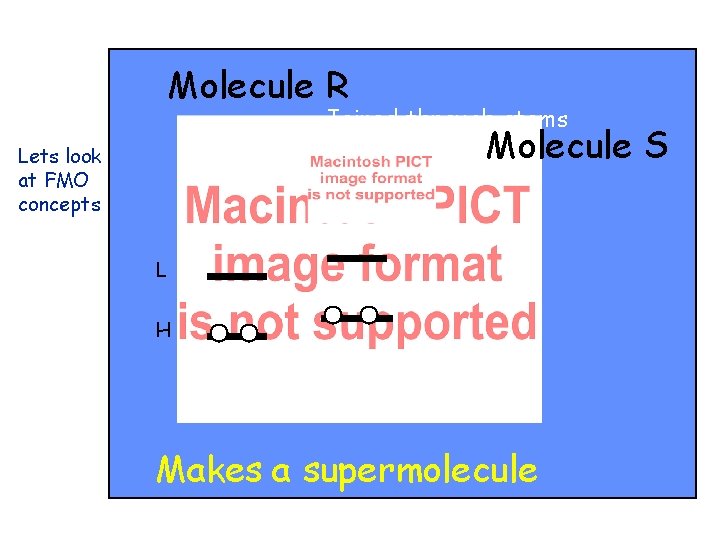
Molecule R Molecule S

Molecule R Joined through atoms r and s Molecule S

Molecule R Joined through atoms r and s Molecule Makes a supermolecule S

The possible interactions Molecule R Joined through atoms r and s Molecule Makes a supermolecule S

Molecule R Joined through atoms r and s Molecule Lets look at FMO concepts L H Makes a supermolecule S

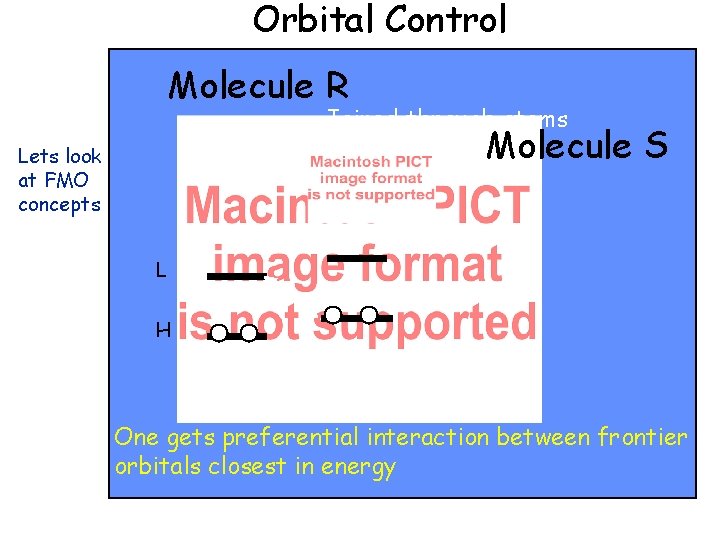
Orbital Control Molecule R Joined through atoms r and s Molecule Lets look at FMO concepts S L H One gets preferential interaction between frontier orbitals closest in energy

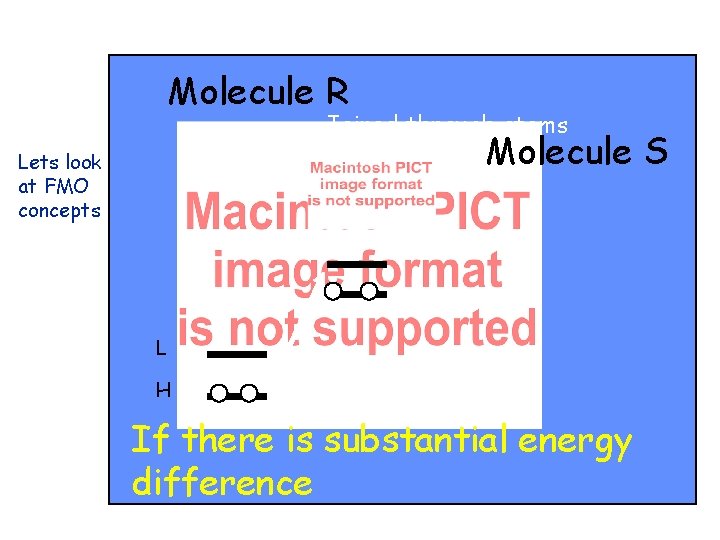
Molecule R Joined through atoms r and s Molecule Lets look at FMO concepts L H If there is substantial energy difference S

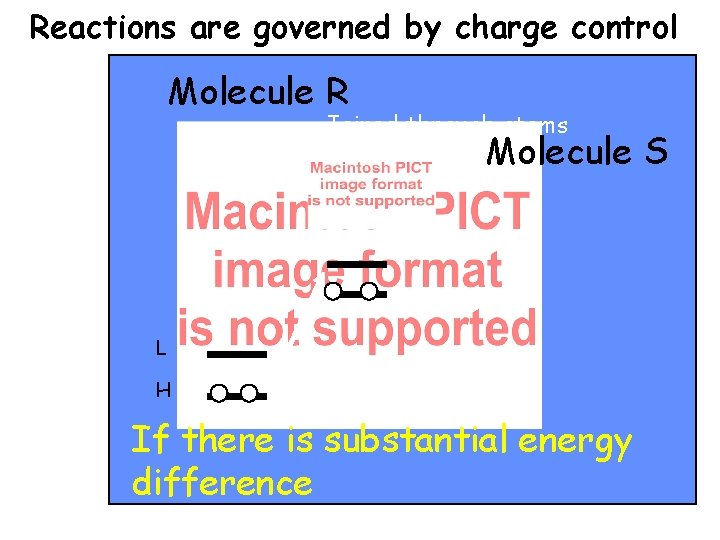
Reactions are governed by charge control Molecule R Joined through atoms r and s Molecule L H If there is substantial energy difference S

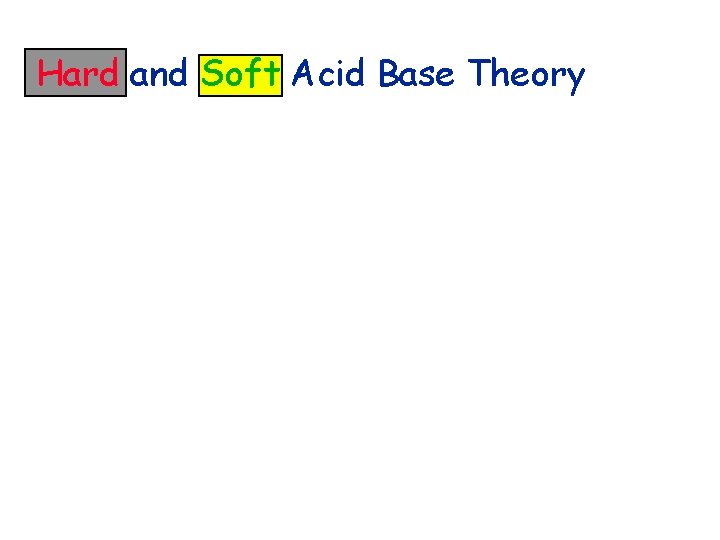
Hard and Soft Acid Base Theory

Hard and Soft Acid Base Theory Charge control involves small, polarizable electron donors and acceptors

Hard and Soft Acid Base Theory Charge control involves small, polarizable electron donors and acceptors Large atoms with little or no charge almost unsovated and readily polarized

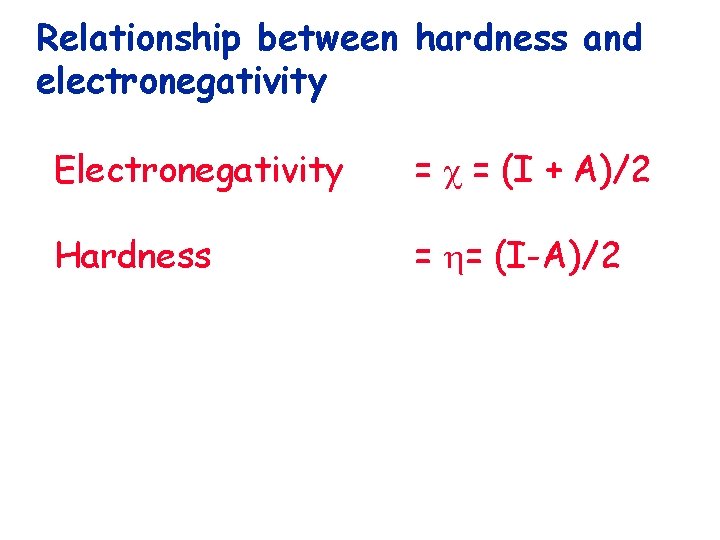
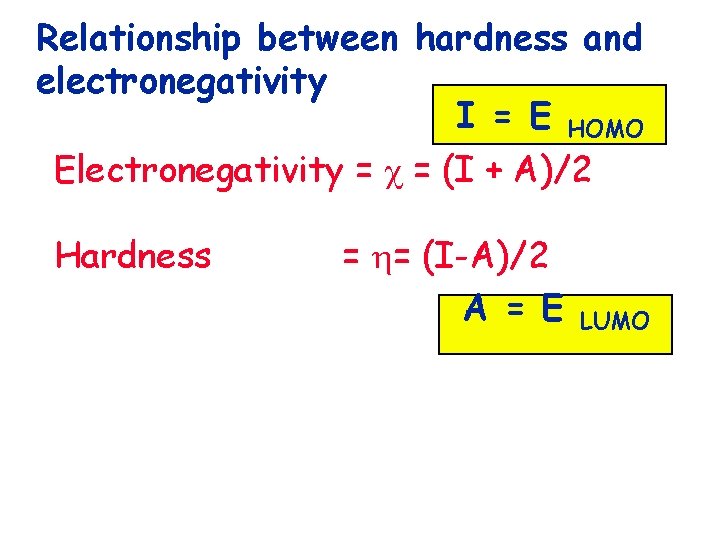
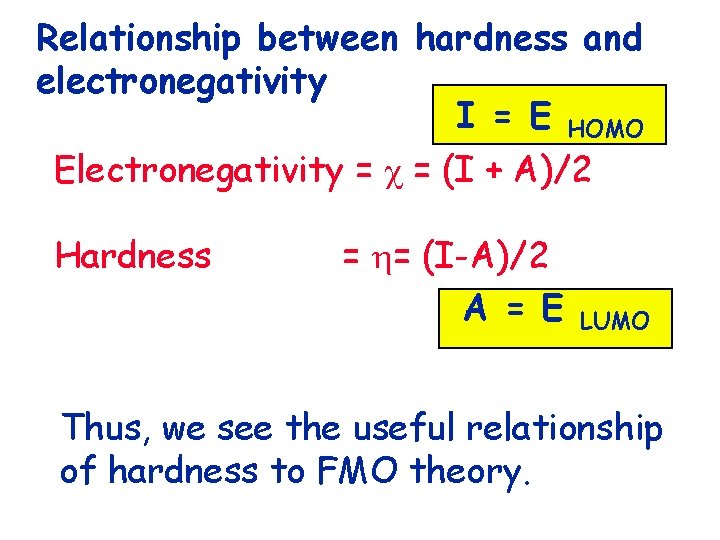
Relationship between hardness and electronegativity
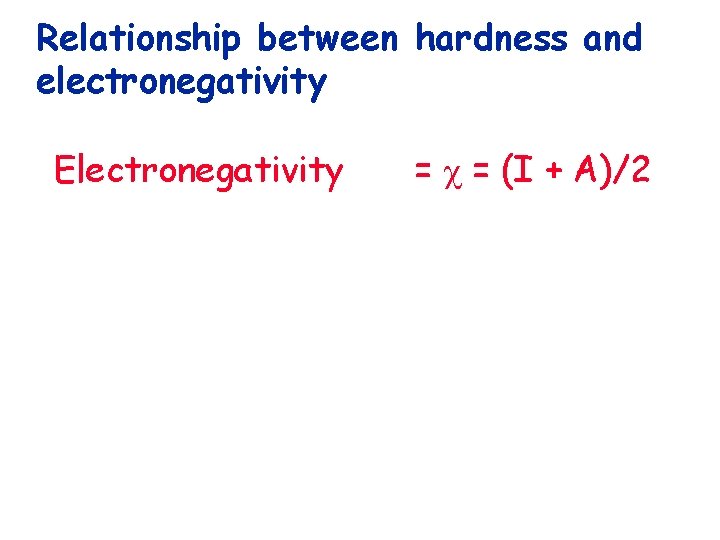
Relationship between hardness and electronegativity Electronegativity = = (I + A)/2

Relationship between hardness and electronegativity Electronegativity = = (I + A)/2 Hardness = = (I-A)/2

Relationship between hardness and electronegativity I = E HOMO Electronegativity = = (I + A)/2 Hardness = = (I-A)/2 A = E LUMO

Relationship between hardness and electronegativity I = E HOMO Electronegativity = = (I + A)/2 Hardness = = (I-A)/2 A = E LUMO Thus, we see the useful relationship of hardness to FMO theory.

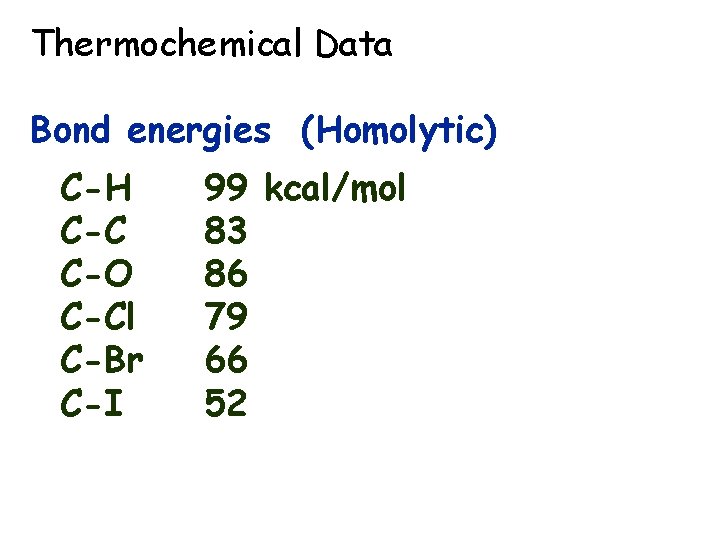
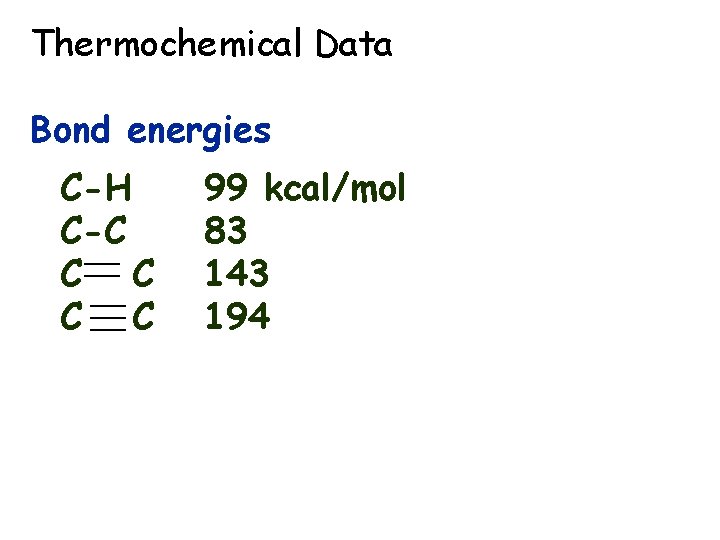
Thermochemical Data Bond energies (Homolytic) C-H C-C C-O C-Cl C-Br C-I 99 kcal/mol 83 86 79 66 52

Thermochemical Data Bond energies (Homolytic) C-H C-C C-O C-Cl C-Br C-I 99 kcal/mol 83 86 79 66 52

Thermochemical Data Bond energies C-H C-C C C 99 kcal/mol 83 143 194

Thermochemical Data Bond energies Try to know some trends:

