How Heat Moves Define Heat Heat is the












- Slides: 14

How ‘Heat’ Moves Define “Heat”: Heat is the movement of thermal energy from a substance at a higher temperature to another substance at a lower temperature. 1

The Nature of Heat moves in only one direction: Under normal conditions and in nature, heat energy will ALWAYS flow the warmer object to the cooler object. Heat energy will flow from one substance to another until the two substances have the same temperature. 2

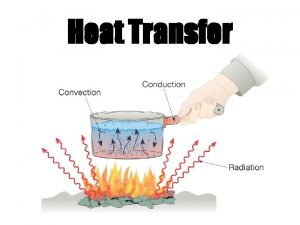
How ‘Heat’ Moves Thermal energy in the form of heat can move in three ways. Conduction Convection Radiation 3

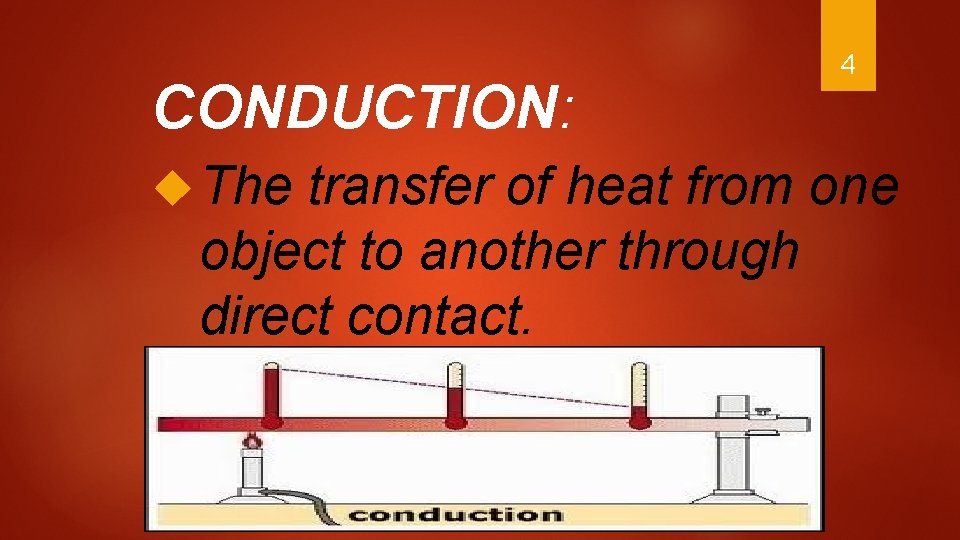
CONDUCTION: The 4 transfer of heat from one object to another through direct contact.

Conduction Example: A metal rod being heated by a flame. 5 A Flame heats one end of the metal rod. b. The heat travels down the rod toward the cooler parts of the metal (away from the fire). c. The temperature increases along the length of the rod, even in parts not touching the flame. a.

Conduction 6 Application: Describe the process of conduction when you place a hot spoon into a bowl of ice cream.

Convection 7 Convection: the transfer of thermal energy (heat) through the movement of a liquid or gas. Thermal Energy heat is carried by the liquid or gas, as it moves from one location to another.

Convection Example: Heating water: When the water at the bottom of the beaker (nearest the burner) is heated, the water absorbs energy by conduction as it touches the bottom. b. The water expands and becomes less dense. c. This causes the heated (hot) water to rise. d. As the hot water rises it loses its energy into the surrounding water and begins to circulate back to the bottom. a. 8

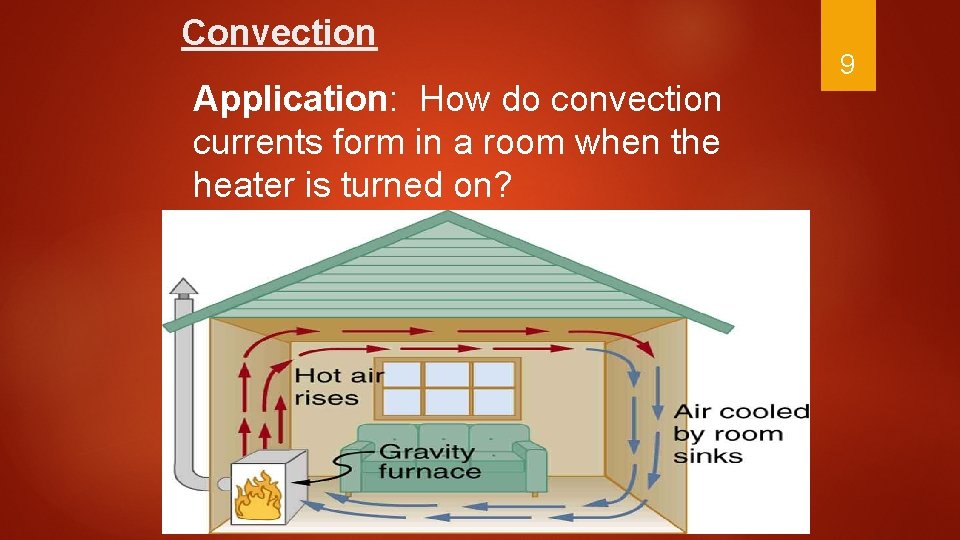
Convection Application: How do convection currents form in a room when the heater is turned on? 9

Radiation Radiation: the transfer of (thermal) energy by electromagnetic waves. Radiation does not require matter to transfer thermal energy. All the sun’s energy that reaches Earth travels through millions of kilometers of empty space (a vacuum). All matter can radiate energy. You feel the radiation of thermal energy from a bonfire, a heat lamp and a light bulb. 10

Radiation Other examples of the transfer of heat by Radiation: a. Charcoal grill. b. Hot tin roof. c. Burner on a stove top. d. ? e. ? 11

Radiation Key Point: For radiation to be felt as heat it must first be absorbed by a material. Example: Why does a black car feel hotter than a white car on a sunny day? 12

The Nature of Heat What happens when you put ice in a warm soft drink? The heat energy moves from the soft drink into the ice by conduction (particle to particle contact) causing the ice to melt. 13

Review Describe three kinds of heat transfer. Conduction – transfer of heat energy from one particle to another by direct contact. (Primarily in solids) b. Convection – transfer of heat energy in fluids-gases and liquids) through the bulk movement of matter from one place to another. (Produces currents) c. Radiation – transfer of energy through electromagnetic waves. (Matter is not required!) (Radiant & infrared radiation from the sun) a. 14
 Heat always moves from
Heat always moves from Heat moves from
Heat moves from Heat moves from
Heat moves from Kinds of heat energy
Kinds of heat energy Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thang điểm glasgow
Thang điểm glasgow Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng