History of the Atom A Ancients Socrates l





- Slides: 11

History of the Atom A. Ancients Socrates l All substances were made up of earth, air, fire and water Democritus l Matter is composed of small particles called “atoms”

B. Dalton l All substances were made up of atoms • Small individual and indestructible parts l MODEL—marble concept • Atom was a small, dense sphere

C. Thomson l Cathode-ray tube experiment • Proved that atoms had negative charges inside them l l Called these charges “electrons” MODEL—”Plum Pudding Model” • Better known as the Chocolate Chip Cookie Model l Cookie is the atom Chips are the electrons http: //www. youtube. com/watch? v=O 9 Goyscbazk

D. Rutherford l Gold-Foil Experiment • When alpha particles passed through gold foil l Proved the atom was mostly empty space • When alpha particles were deflected l Proved atoms had a small, dense core • Called this area a nucleus which contained positive particles called protons • http: //www. youtube. com/watch? v=U 83 swkc. L 32 w

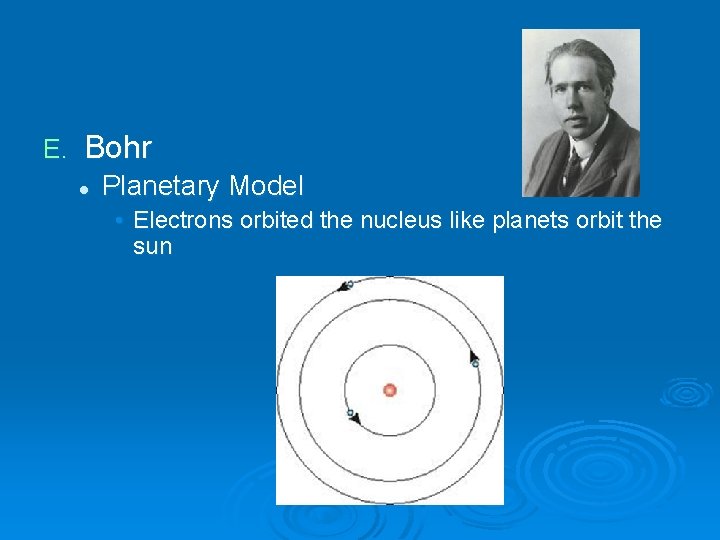
E. Bohr l Planetary Model • Electrons orbited the nucleus like planets orbit the sun

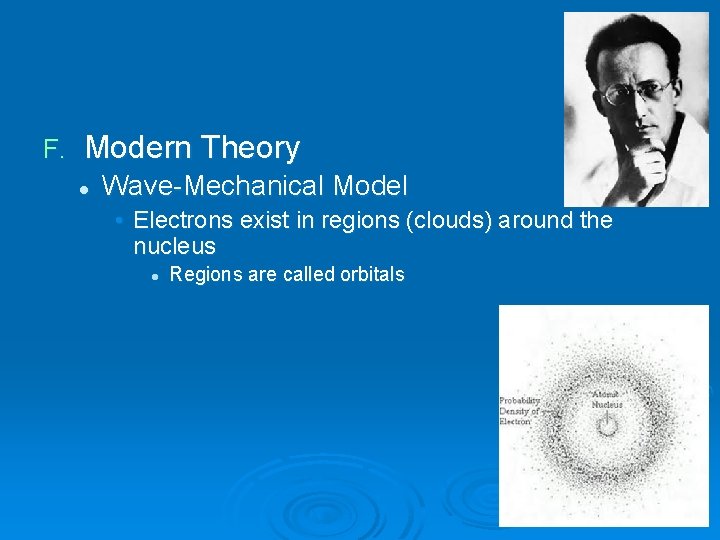
F. Modern Theory l Wave-Mechanical Model • Electrons exist in regions (clouds) around the nucleus l Regions are called orbitals

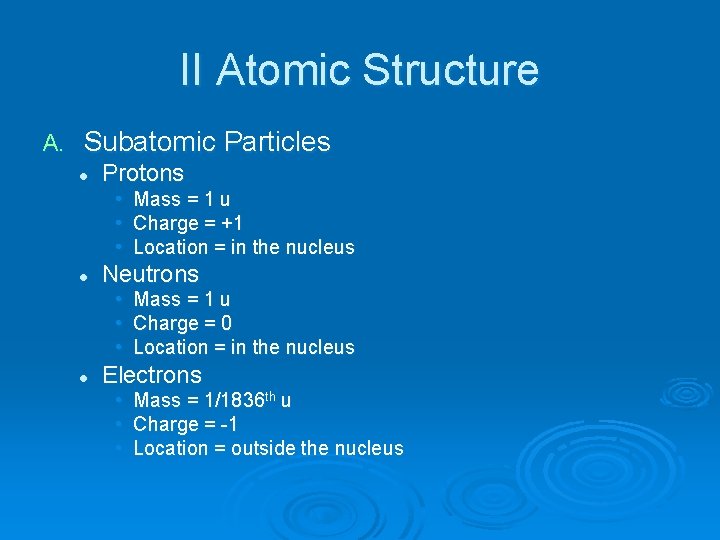
II Atomic Structure A. Subatomic Particles l Protons • Mass = 1 u • Charge = +1 • Location = in the nucleus l Neutrons • Mass = 1 u • Charge = 0 • Location = in the nucleus l Electrons • • • Mass = 1/1836 th u Charge = -1 Location = outside the nucleus

B. Atomic Number l l Represents the number of protons in an atom Identifies the element • Change the atomic number—change the element l Can also be used to determine the number of electrons • Atom l Protons = electrons • Ion l Changes the number of electrons • (+) ion loses electrons • (-) ions gains electrons

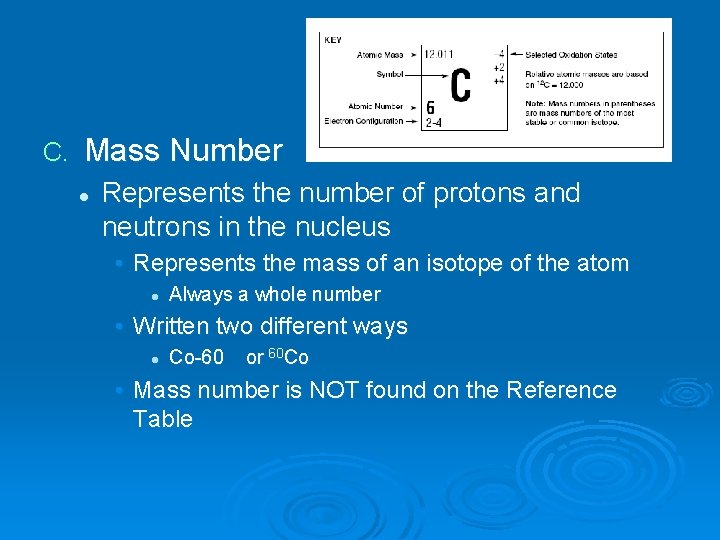
C. Mass Number l Represents the number of protons and neutrons in the nucleus • Represents the mass of an isotope of the atom l Always a whole number • Written two different ways l Co-60 or 60 Co • Mass number is NOT found on the Reference Table

1. Isotope l An isotope is a different form of the same element • Neutrons change • Mass number changes l Example C-12 and C-14 • C-12 protons = 6 neutrons = 6 • C-14 protons = 6 neutrons = 8 • C-12 is stable while C-14 is radioactive

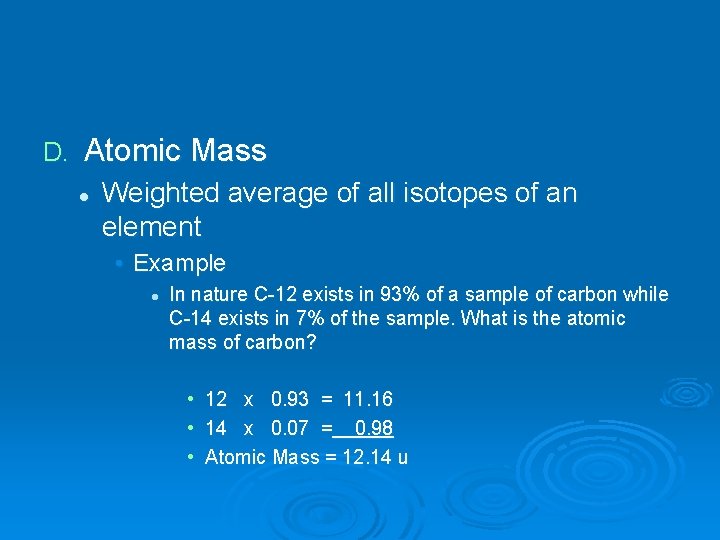
D. Atomic Mass l Weighted average of all isotopes of an element • Example l In nature C-12 exists in 93% of a sample of carbon while C-14 exists in 7% of the sample. What is the atomic mass of carbon? • • • 12 x 0. 93 = 11. 16 14 x 0. 07 = 0. 98 Atomic Mass = 12. 14 u
 Fatiguibility
Fatiguibility A student of socrates he wrote down his
A student of socrates he wrote down his The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Perkembangan model atom dalton
Perkembangan model atom dalton Atomic history webquest
Atomic history webquest How many protons does oxygen have
How many protons does oxygen have Democritus atomic model
Democritus atomic model History of the atom john dalton
History of the atom john dalton History of the atom democritus
History of the atom democritus Atom models history
Atom models history Atoms graphic organizer
Atoms graphic organizer Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể