History of Mineralogy Earth Structure History of Mineralogy



















- Slides: 41

History of Mineralogy & Earth Structure

History of Mineralogy Gold Silver Copper Iron Tin Lead Sulfur Mercury Carbon Nine elements were known from prehistory

History of Mineralogy • Stone age cave paintings show mineral use – Fe and Mn oxides • Ancient Egyptians traded gems and metals, and did smelting

History of Mineralogy • Theophrastus – ~371 -287 BC – Wrote the first book on mineralogy, On Stones • Work focused on the effect of heat on different stones and minerals, and other distinguishing properties – Also wrote foundational books on botany

History of Mineralogy • Pliny the Elder – 23 -79 AD – Wrote Naturalis Historia, about rocks minerals and metals found near Rome, focused on economic uses. • Laid groundwork for crystallography • Recognized amber as fossilized resin – Wrote a detailed, eye-witness description of the eruption of Mt. Vesuvius • He died during a selfrescue mission directed

History of Mineralogy • Georgius Agricola – Published De Re Metallica, 1556 – Described mining and smelting practices of the day – First detailed, qualitative accounts of minerals

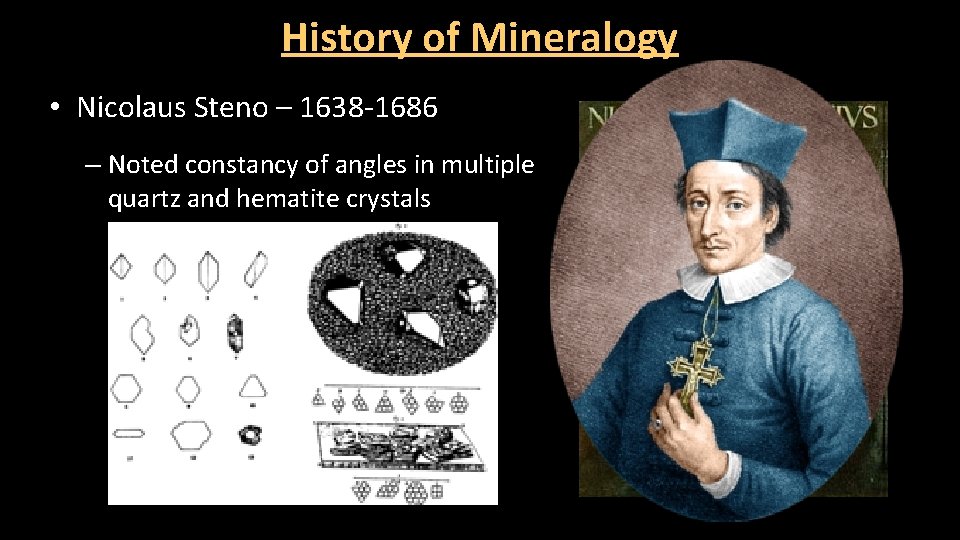
History of Mineralogy • Nicolaus Steno – 1638 -1686 – Noted constancy of angles in multiple quartz and hematite crystals

History of Mineralogy • Arnould Carangeot – 1742 -1806 – Invented the contact goniometer in 1780 – Made measurements of angles between crystal faces easier

History of Mineralogy • Jean-Baptiste Romé de l’Isle – 1736 -1790 – Made measurements of angles between crystal faces that confirmed Steno’s previous work – Formally stipulated the “Law of the Constancy of Interfacial Angles. ”

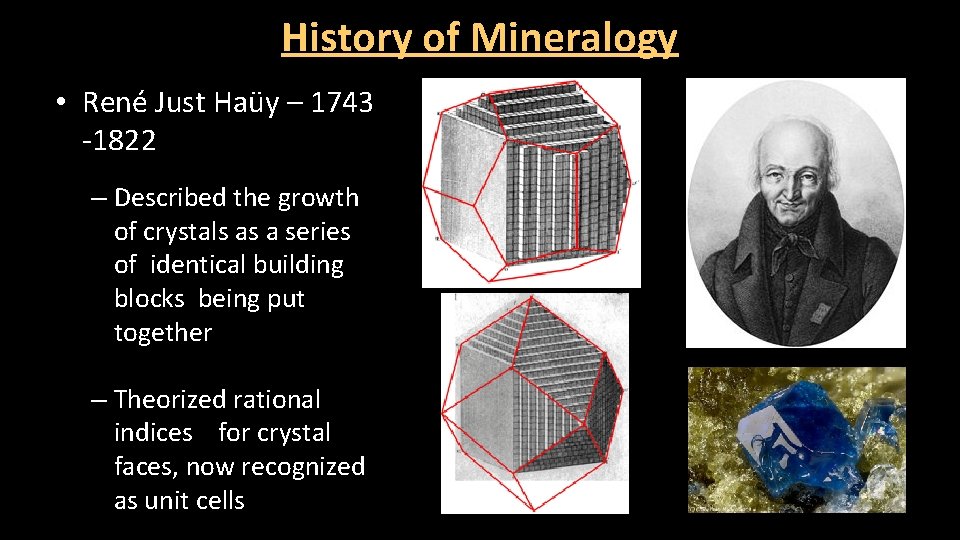
History of Mineralogy • René Just Haüy – 1743 -1822 – Described the growth of crystals as a series of identical building blocks being put together – Theorized rational indices for crystal faces, now recognized as unit cells

History of Mineralogy • William Hyde Wollaston – 1766 -1828 – Invented the reflecting goniometer • First goniometer to use mirrors for measuring angles between faces – Allowed more precise crystal face measurements

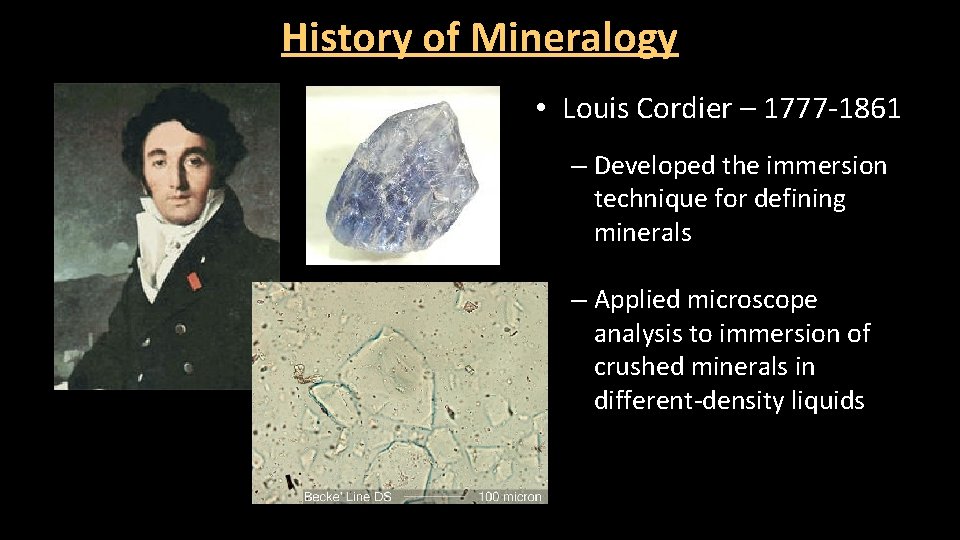
History of Mineralogy • Louis Cordier – 1777 -1861 – Developed the immersion technique for defining minerals – Applied microscope analysis to immersion of crushed minerals in different-density liquids

History of Mineralogy • William Nicol – 1770 -1851 – Invented the polarizing microscope in 1828 – Allowed for the study of light refraction in crystals – Employs the use of two polarizing lenses along the line of sight, rotated at 90° to each other

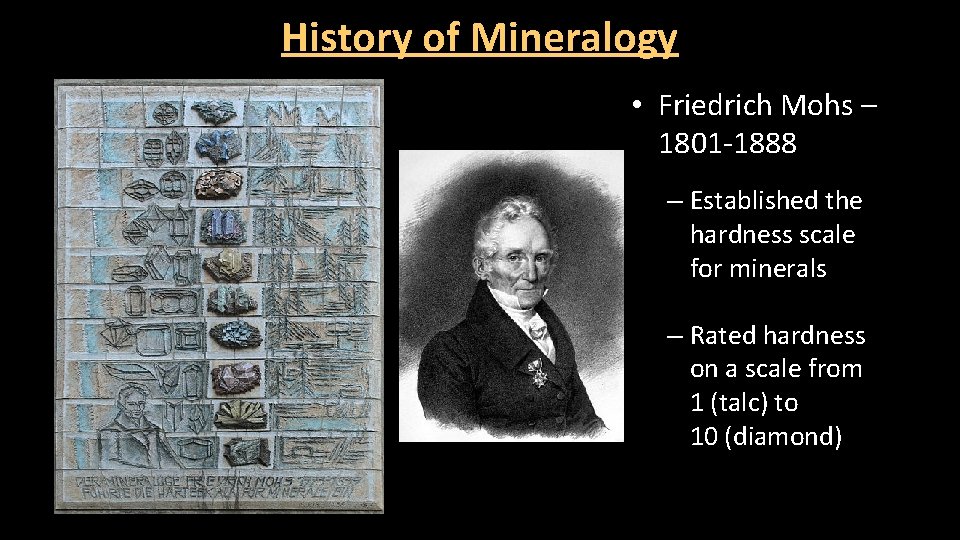
History of Mineralogy • Friedrich Mohs – 1801 -1888 – Established the hardness scale for minerals – Rated hardness on a scale from 1 (talc) to 10 (diamond)

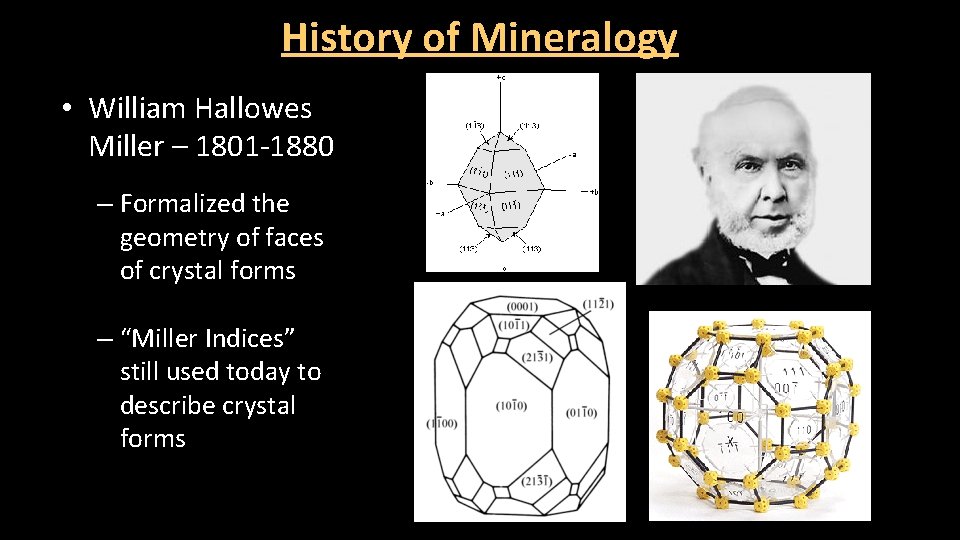
History of Mineralogy • William Hallowes Miller – 1801 -1880 – Formalized the geometry of faces of crystal forms – “Miller Indices” still used today to describe crystal forms

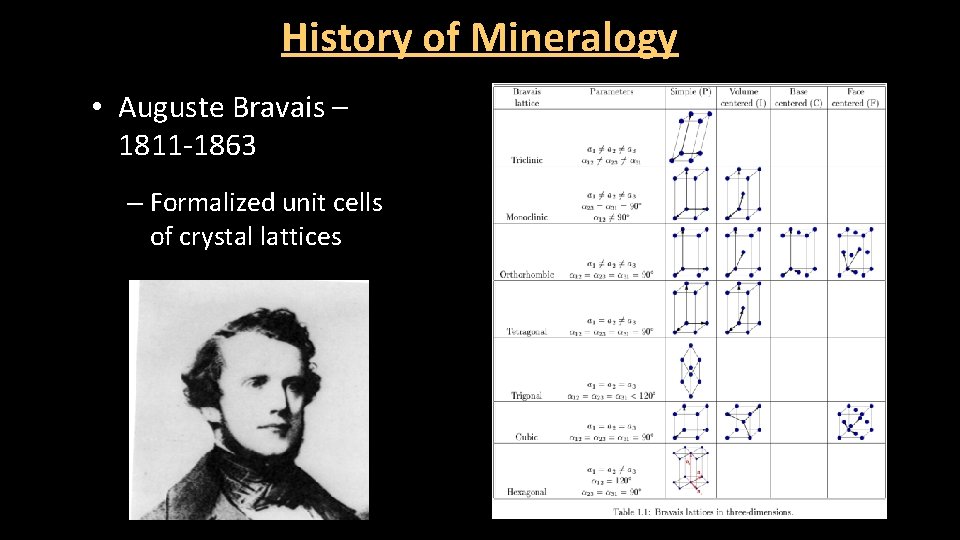
History of Mineralogy • Auguste Bravais – 1811 -1863 – Formalized unit cells of crystal lattices

History of Mineralogy • James D. Dana – 1813 -1895 – Formalized the systematic classification of minerals • “System of Mineralogy” 1837 • “Manual of Mineralogy” 1848 • “Manual of Geology” 1863 – Our own textbook, “Manual of Mineral Science” is the 23 rd revised edition of Dana’s original text – The Dana Medal in mineralogy is named in his honor

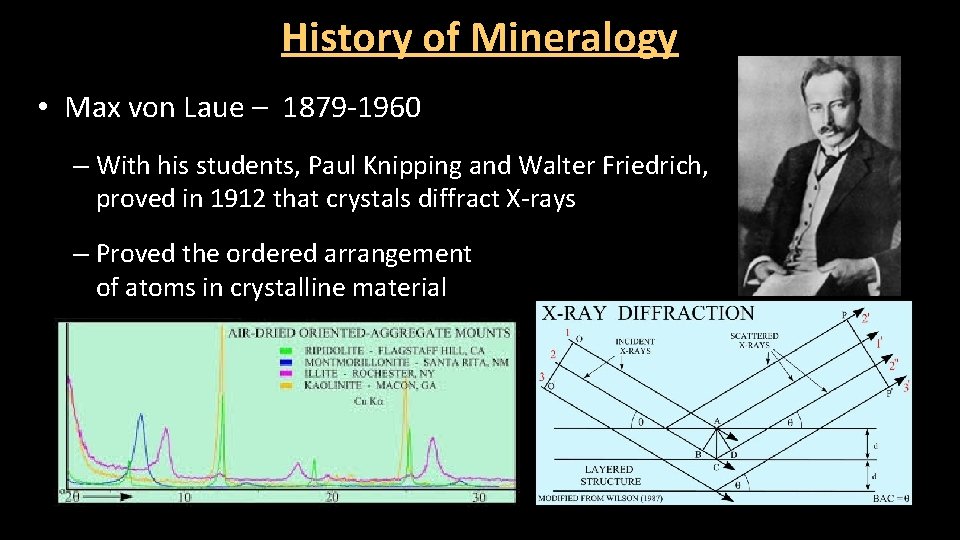
History of Mineralogy • Max von Laue – 1879 -1960 – With his students, Paul Knipping and Walter Friedrich, proved in 1912 that crystals diffract X-rays – Proved the ordered arrangement of atoms in crystalline material

History of Mineralogy • William H. Bragg (1862 -1942) and William L. Bragg (1890 -1971) – Published determinations of crystal structure based on x-ray diffraction – Joint winners of 1915 Nobel Prize in Physics

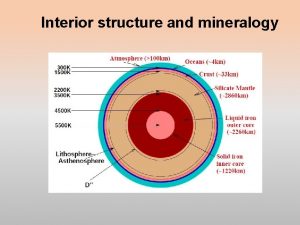
Interior of the Earth Two ways to define the layers Chemical Properties Physical Properties

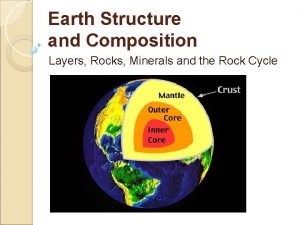
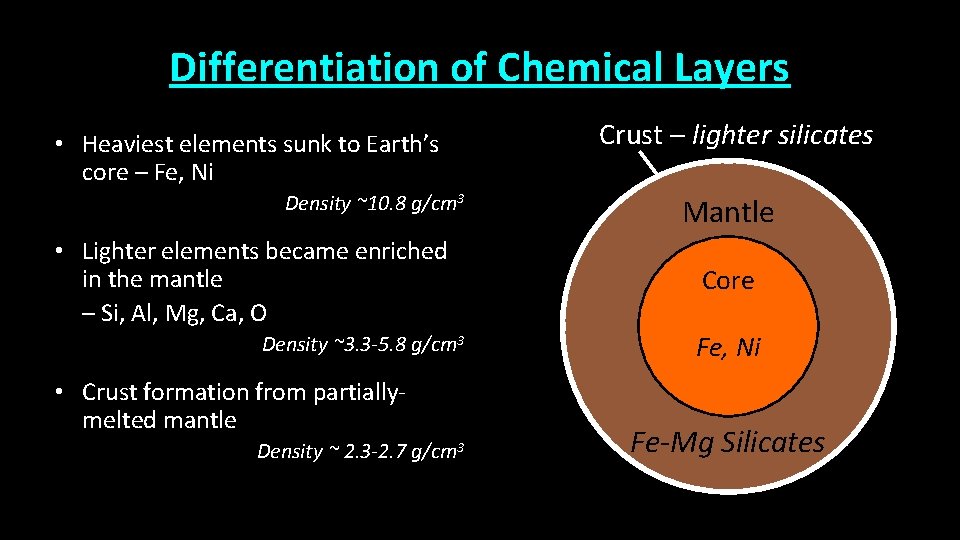
Differentiation of Chemical Layers • Heaviest elements sunk to Earth’s core – Fe, Ni Density ~10. 8 g/cm 3 • Lighter elements became enriched in the mantle – Si, Al, Mg, Ca, O Density ~3. 3 -5. 8 g/cm 3 • Crust formation from partiallymelted mantle Density ~ 2. 3 -2. 7 g/cm 3 Crust – lighter silicates Mantle Core Fe, Ni Fe-Mg Silicates

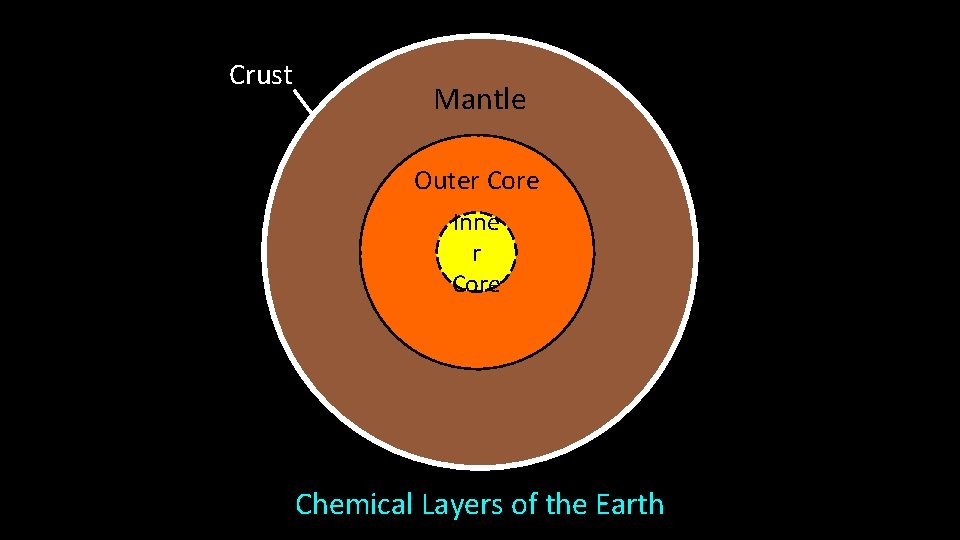
Crust Mantle Outer Core Inne r Core Chemical Layers of the Earth

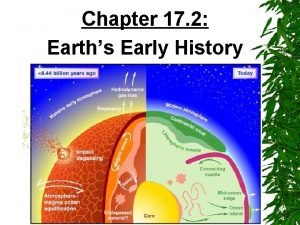
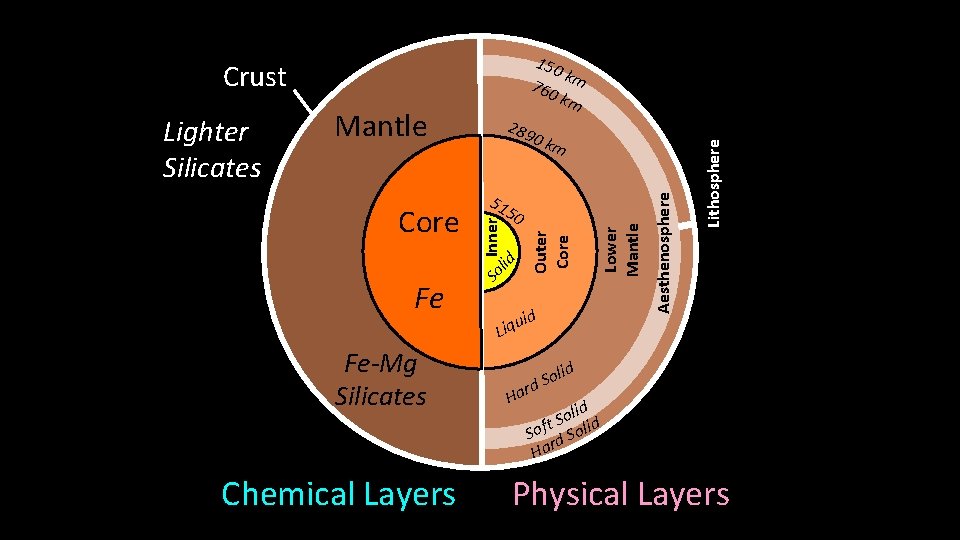
Chemical Layers of Earth: Crust (two types): rich in Si, Al, Ca, O • Continental (20 -70 km; avg = 35 km) • Oceanic (0 -20 km; avg = 6 km) Mantle: rich in Mg, Fe, Si, O • Base of crust (6 -35 km) to 2900 km depth • Partially melted, NOT liquid Core: metallic Fe • Outer liquid core (2900 - 5150 km) • Inner solid core (5150 - 6370 km)

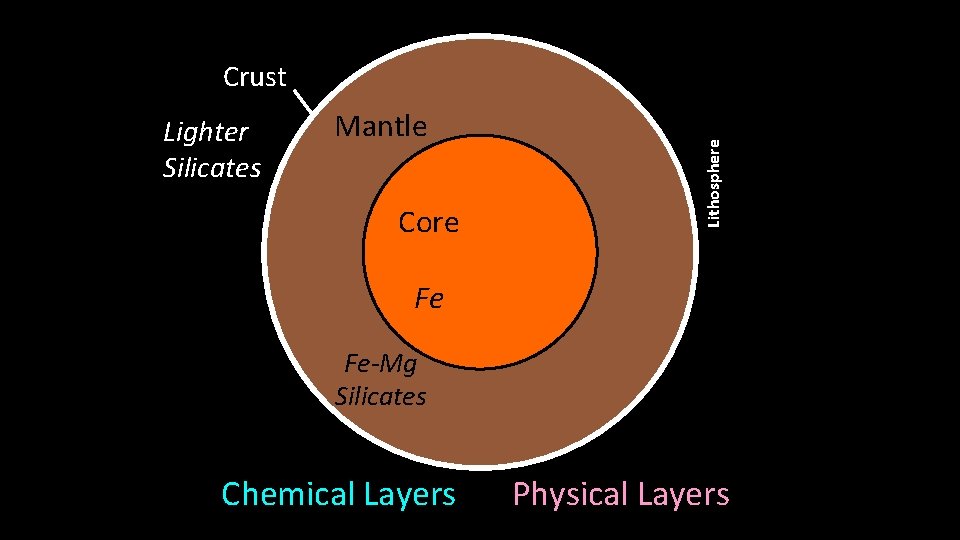
Mantle 289 0 km Outer Core Fe rnn Core Outer Core So Inner lid Core. Inne 50 Fe-Mg Silicates Chemical Layers Lower Mantle 51 d ui Liq Lithosphere Lighter Silicates Mantle Aesthenosphere Crust 150 k 760 m km olid S d Har lid o S t d Sof d Soli Har Physical Layers

Mantle 289 0 km Outer Core Fe rnn Core Outer Core So Inner lid Core. Inne 50 Fe-Mg Silicates Chemical Layers Lower Mantle 51 d ui Liq Lithosphere Lighter Silicates Mantle Aesthenosphere Crust 150 k 760 m km olid S d Har lid o S t d Sof d Soli Har Physical Layers

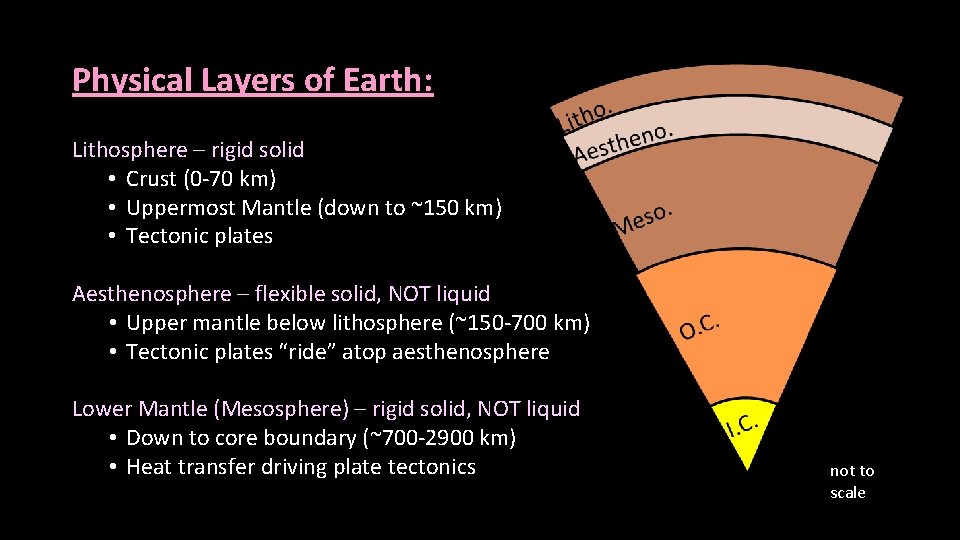
Physical Layers of Earth: Lithosphere – rigid solid • Crust (0 -70 km) • Uppermost Mantle (down to ~150 km) • Tectonic plates Aesthenosphere – flexible solid, NOT liquid • Upper mantle below lithosphere (~150 -700 km) • Tectonic plates “ride” atop aesthenosphere Lower Mantle (Mesosphere) – rigid solid, NOT liquid • Down to core boundary (~700 -2900 km) • Heat transfer driving plate tectonics not to scale

Physical Layers of Earth: Outer Core – rotating, molten Fe-alloy • Base of mantle to inner core (2900 - 5100 km) • Source of Earth’s electromagnetic field • Liquid due to ultra-high temperature Inner Core – solid Fe-alloy • Depth: 5100 - 6370 km • Solid due to overriding effect of pressure How do we know what’s down there? How do we know the boundaries? not to scale

How do we know what’s down there? You can’t go to the mantle (or the core) – Deepest we’ve ever drilled is only ~12 km – Earth’s continental crust is ~30 -70 km thick – Core/Mantle boundary 2900 km below surface

What’s down there? Planet parts come to us – Mantle xenoliths show mineralogy of deep Earth – Meteorites can provide evidence of iron cores and “rocky” outer layers

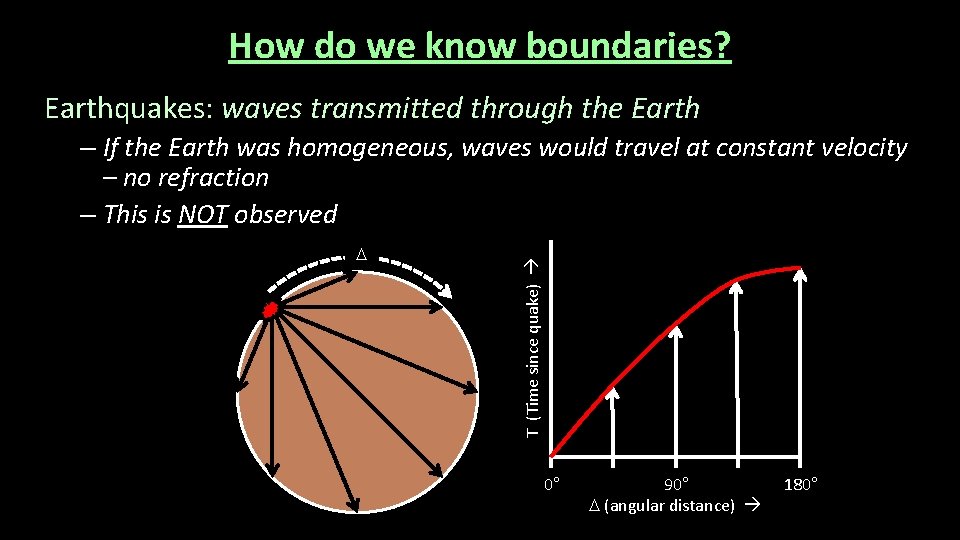
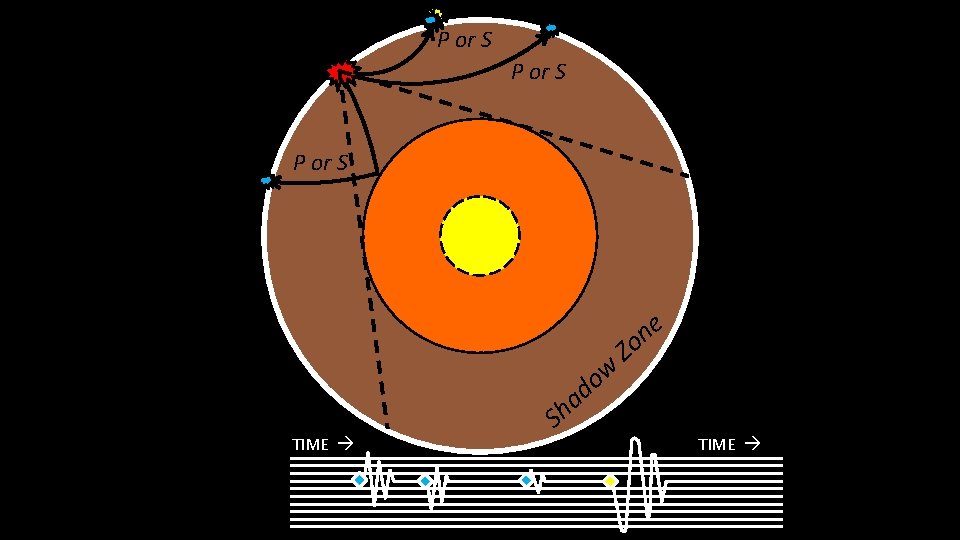
How do we know boundaries? Earthquakes: waves transmitted through the Earth D T (Time since quake) – If the Earth was homogeneous, waves would travel at constant velocity – no refraction – This is NOT observed 0° 90° D (angular distance) 180°

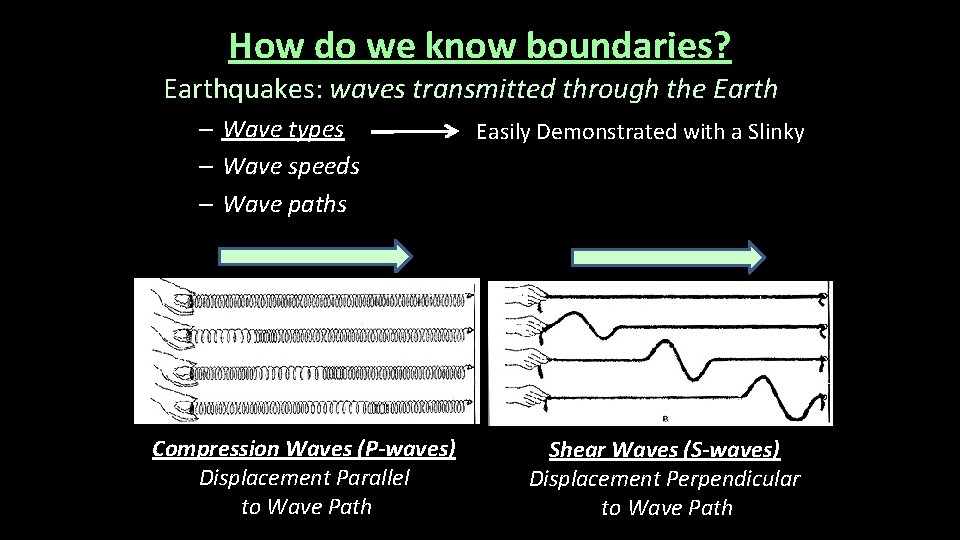
How do we know boundaries? Earthquakes: waves transmitted through the Earth – Wave types – Wave speeds – Wave paths Compression Waves (P-waves) Displacement Parallel to Wave Path Easily Demonstrated with a Slinky Shear Waves (S-waves) Displacement Perpendicular to Wave Path

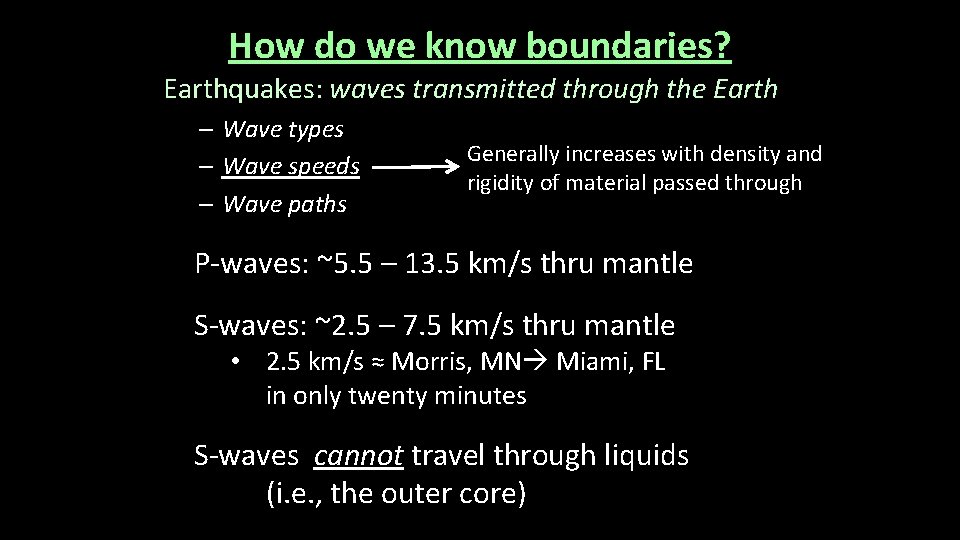
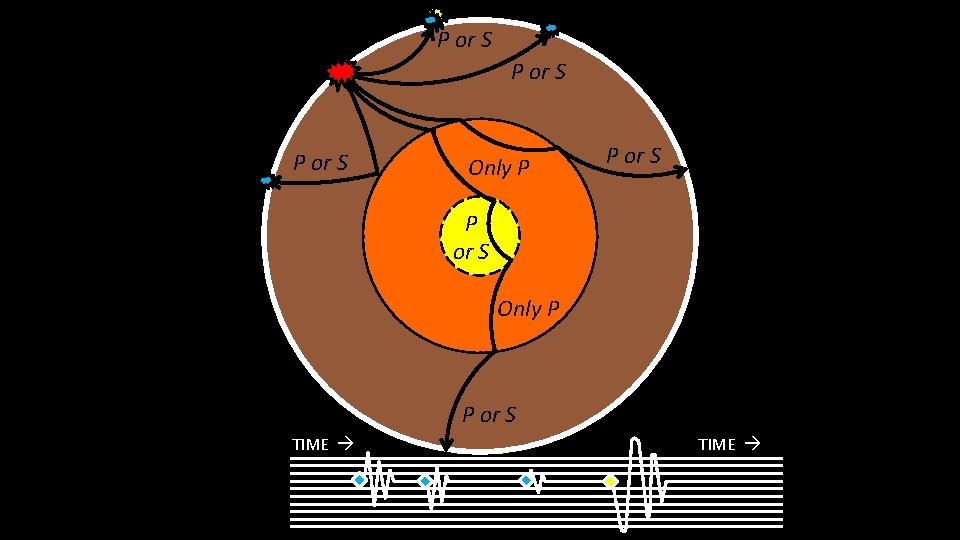
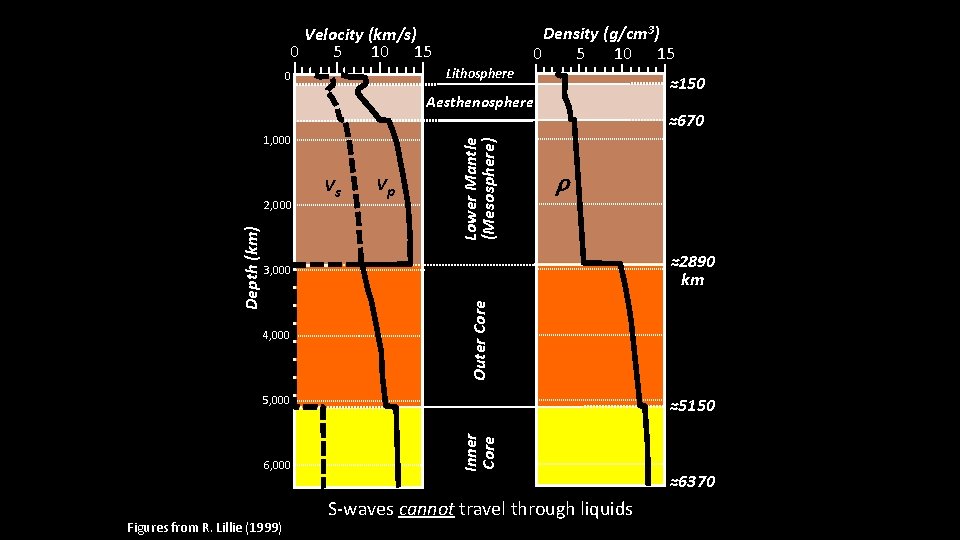
How do we know boundaries? Earthquakes: waves transmitted through the Earth – Wave types – Wave speeds – Wave paths Generally increases with density and rigidity of material passed through P-waves: ~5. 5 – 13. 5 km/s thru mantle S-waves: ~2. 5 – 7. 5 km/s thru mantle • 2. 5 km/s ≈ Morris, MN Miami, FL in only twenty minutes S-waves cannot travel through liquids (i. e. , the outer core)

How do we know boundaries? Earthquakes: waves transmitted through the Earth – Wave types – Wave speeds – Wave paths Study seismograms Define wave arrival times Where is the wave reflected? Where is the wave refracted? Where is the energy transmitted as a P-wave? Where is the energy transmitted as an S-wave?

P or S w o d TIME a h S e n Zo TIME

P or S Only P P or S TIME

Density (g/cm 3) 5 0 10 15 Velocity (km/s) 5 0 10 15 Lithosphere 0 ≈150 1, 000 Vp ≈670 r ≈2890 km 3, 000 4, 000 Outer Core Depth (km) 2, 000 Vs Lower Mantle (Mesosphere) Aesthenosphere 5, 000 Figures from R. Lillie (1999) Inner Core 6, 000 ≈5150 S-waves cannot travel through liquids ≈6370

Recap: What are Minerals? • The building blocks of rocks • Definition – Naturally occurring – Solid – Inorganic – Crystalline – Definite chemical formula • Rocks are typically aggregates of minerals (they may contain many different minerals or very few)

History of Mineralogy, Summarized • Ancient and Medieval focus on describing, classifying, and using pure elements, metals, and gems • Efforts during Renaissance and Enlightenment to quantify crystal forms and structures using goniometers and microscopic analyses

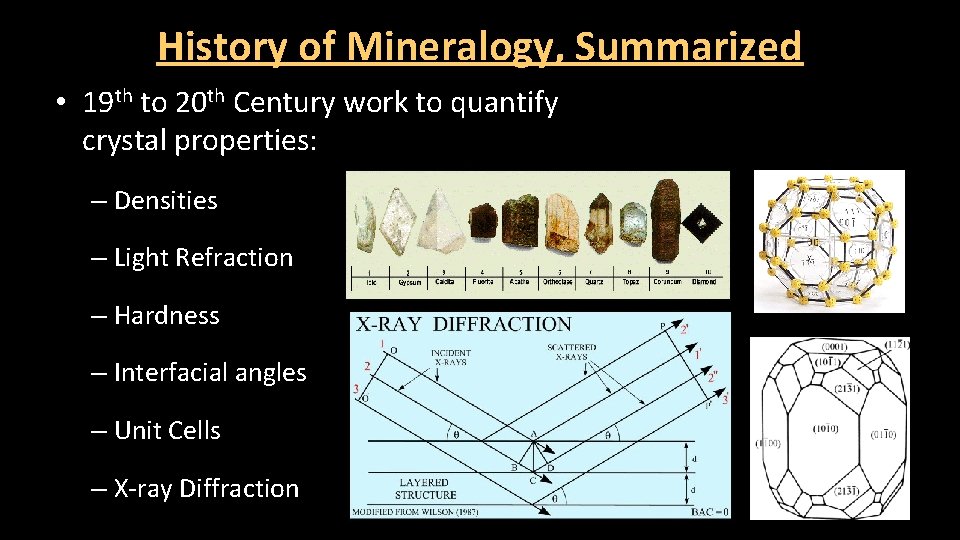
History of Mineralogy, Summarized • 19 th to 20 th Century work to quantify crystal properties: – Densities – Light Refraction – Hardness – Interfacial angles – Unit Cells – X-ray Diffraction

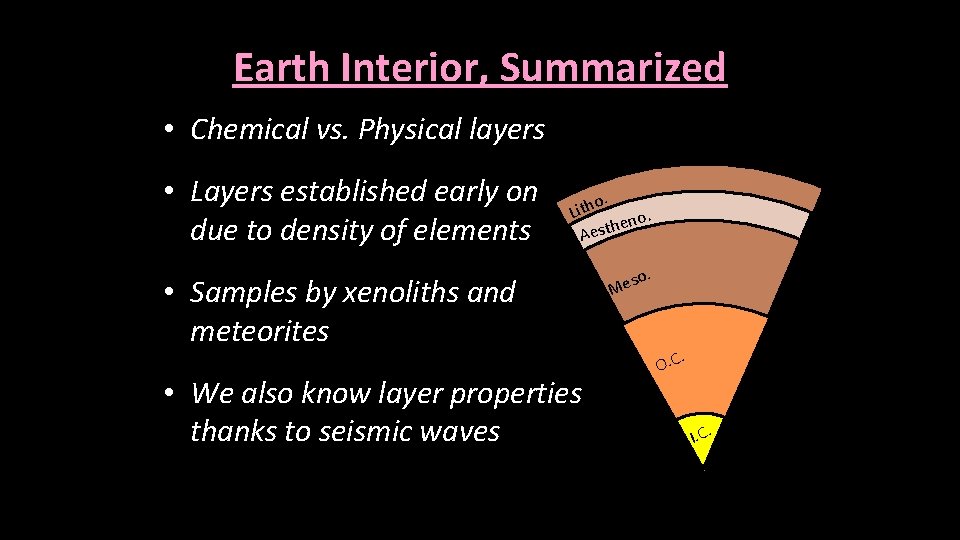
Earth Interior, Summarized • Chemical vs. Physical layers • Layers established early on due to density of elements o. Lith no. e h t s Ae • Samples by xenoliths and meteorites • We also know layer properties thanks to seismic waves . so Me . O. C I. C.

Next Time… • Physical Properties of Minerals – How do we describe minerals? – Why do they have these properties?
 Accessory plate microscope
Accessory plate microscope Melatope mineralogy
Melatope mineralogy Isotropic minerals
Isotropic minerals Extinction angle of minerals
Extinction angle of minerals Mineralogy lab
Mineralogy lab Isochromes
Isochromes Mineralogy
Mineralogy Kyanite formula
Kyanite formula Optical mineralogy
Optical mineralogy Clock analogy of earth's history
Clock analogy of earth's history Section 17-2 earth's early history
Section 17-2 earth's early history 17-2 earth's early history
17-2 earth's early history Clock analogy of earth's history
Clock analogy of earth's history Define serial endosymbiosis
Define serial endosymbiosis Section 17-2 earth's early history
Section 17-2 earth's early history History of the earth
History of the earth Chapter 25 the history of life on earth
Chapter 25 the history of life on earth Graphic organizer geologic time scale
Graphic organizer geologic time scale History of life on earth
History of life on earth Earth history
Earth history Tuzo wilson
Tuzo wilson Faith reason and earth history pdf
Faith reason and earth history pdf Vertical structure of the atmosphere lab 1
Vertical structure of the atmosphere lab 1 Earth structure
Earth structure Layers of the earth convection currents
Layers of the earth convection currents Earth structure
Earth structure Earth internal structure
Earth internal structure Earth internal structure
Earth internal structure Group 13 elements
Group 13 elements Dynamic earth structure
Dynamic earth structure Mercury barometer
Mercury barometer Structure of the earth
Structure of the earth Also history physical
Also history physical How to structure a 4 mark history question
How to structure a 4 mark history question Chapter 5 policing history and structure
Chapter 5 policing history and structure Gcse history questions and answers
Gcse history questions and answers Gcse history how useful questions
Gcse history how useful questions How to write a higher history essay
How to write a higher history essay Physical state of covalent compounds
Physical state of covalent compounds Union myunion structure my structure integer m float n
Union myunion structure my structure integer m float n Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Structural ambiguity examples
Structural ambiguity examples