Hemophagocytic lymphohistiocytosis in nontransplant setting Report of two

















- Slides: 31

Hemophagocytic lymphohistiocytosis in non-transplant setting: Report of two cases and the treatment challenges 1 Hadi Mottaghi Pisheh Pediatric Hematology & oncology Fellowship Shiraz University of Medical Sciences Kish Island- November 2018 hmottaghipisheh@gmail. com

2 Background and Objective Uncommon hyper-inflammatory syndrome Associated with different conditions, including neoplastic, infectious, autoimmune, or hereditary diseases Grouped into two forms: Familial type (genetic mutations affecting the cytotoxic function of T lymphocytes and natural killer (NK) cells) Acquired form presenting in different conditions such as infectious, malignant, rheumatologic, or metabolic diseases.

Background and Objective 3 Hematopoietic stem cell transplantation (HSCT) is the recommended treatment for patients with: familial HLH CNS involvement recurrent/refractory disease. .

4 Background and Objective Herein, we present two typical cases of HLH who were strict candidates of HSCT with no appropriate HLA-matched donor worldwide. The big challenge is : how long they should be treated with maintenance chemotherapy what will be the best treatment option

5 Case 1: An 8 -year-old boy was admitted to the hematology department in Amir Hospital (Shiraz, Iran) due to clinical findings such as fever, jaundice, hepatosplenomegaly, and pancytopenia. Other laboratory findings: an increased level AST, ALT, LDH, Bilirubin, PTT high serum ferritin (nearly 1000 ng/ml) low level of fibrinogen. high titer of Ebstein-Barr virus (EBV) viral capsid antigen Ig. M antibody.

6 Hepatitis A, B, and C infections, HIV infection, auto immune hepatitis and Wilson disease were ruled out by appropriate tests. Liver biopsy bone marrow aspiration and biopsy was inconclusive with nonspecific findings. Laboratory tests such as NK cells function and s. CD 25 was not checked as they were not available in our center. The patient was suspected as a case of HLH according to HLH-2004 protocol The diagnosis was confirmed with NGS which covered the entire coding exons which revealed a homozygous missense mutation in PRF 1 gene.

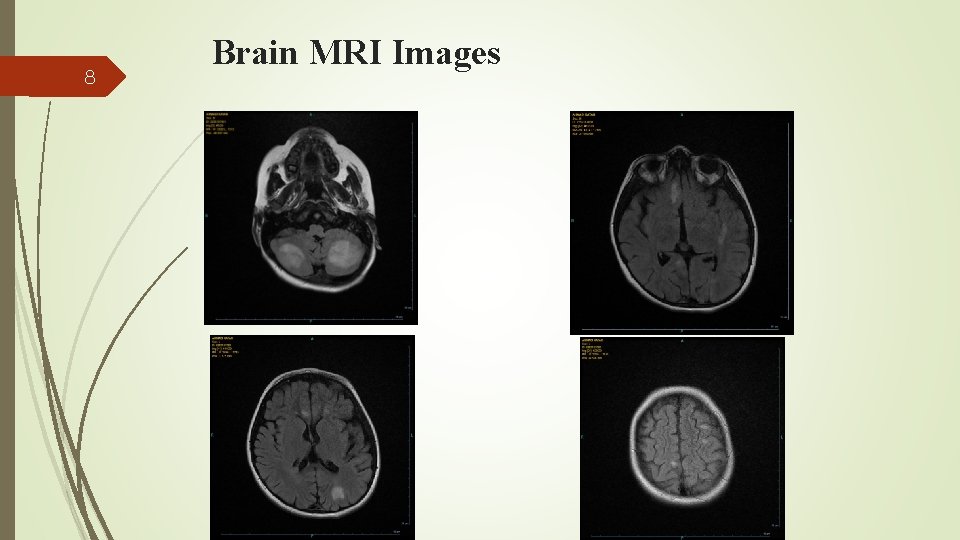
7 He was treated with HLH-2004 protocol with Dramatic response. CNS involvement during treatment course presenting with convulsion, ataxia, spasticity and slurred speech. CSF analysis for cell count, protein and cytology were normal. Spots of white matter hyper signal intensities on T 2 and FLAIR images brain MRI Intrathecal (IT) methotrexate and hydrocortisone was added to his treatment regimen

8 Brain MRI Images

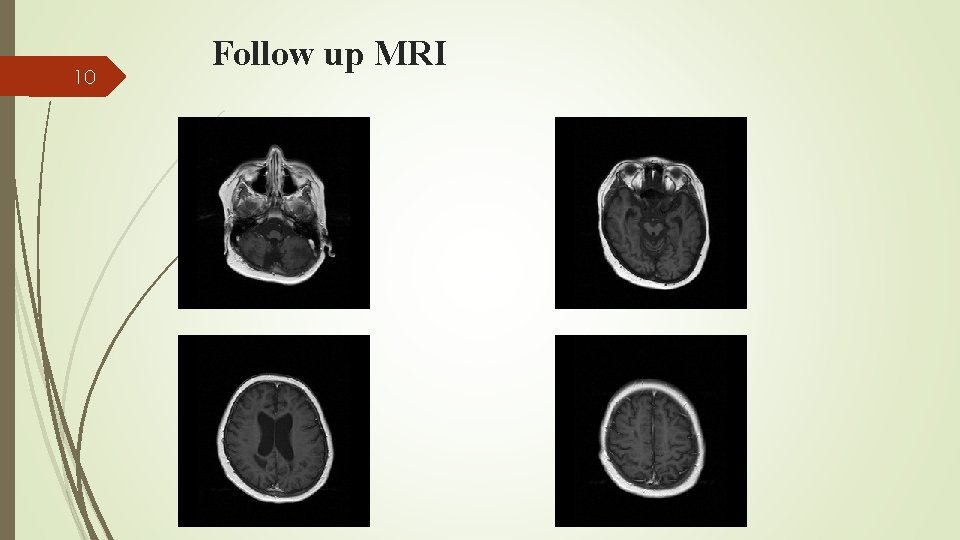
9 Global search for an HLA-matched donor for HSCT revealed no suitable donor. Maintenance treatment with cyclosporine, intermittent monthly dexamethasone and IT methotrexate and hydrocortisone was continued His CNS symptoms were initially progressive and now stable disease. Diffuse cortical atrophy in follow up MRI Currently, no systemic or laboratory evidence of HLH reactivation, and is still waiting for a suitable stem cell donor.

10 Follow up MRI

11 Case 2: An 8 -month-old boy was admitted to the hematology department in Amir Hospital (Shiraz, Iran) due to prolonged fever and hepatosplenomegaly for a 2 -month duration. Laboratory findings: Increased level of AST, ALT, LDH, serum ferritin (>800 ng/ml) pancytopenia high level of triglyceride Persistently low level of Ig. G.

12 Serological markers for hepatitis A, B, and C and EBV were negative, and antibody titers for autoimmune hepatitis were within normal range. Bone marrow aspiration and biopsy was inconclusive with nonspecific findings. Laboratory tests such as defective killing activity of either CD 8 or NK cells and soluble CD 25 were not available in our center. Moreover, no known genetic mutation involving FHL genes was founded by NGS method. The patient was suspected as a case of HLH according to HLH-2004 protocol

13 Treatment according to HLH-2004 protocol was started and continued for 40 weeks complete resolution of clinical and laboratory findings of HLH. There was no clinical, laboratory or radiologic evidence of CNS involvement both initially and during treatment monitoring. He was off treatment for about one year. He returned with reactivation of his disease.

14 Treated with the same protocol due to good response in the first episode He was also treated with monthly IVIG. As we found no suitable stem cell donor in the global search, his treatment continued for 40 weeks. Since then, he is on daily cyclosporine and intermittent doses of monthly dexamethasone for 5 consecutive days. We are still waiting for an HLA-matched stem cell donor

15 Discussion 1 st case with familial HLH and CNS involvement It would be too risky to discontinue maintenance therapy given he is at high risk of disease flare up. However, IT injection of cytotoxic agents will be accompanied by long-term sequels and will impact the quality of life. Repeated exposure with chemotherapy agents especially etoposide will increase the risk of second malignant neoplasm. Mismatched unrelated donor is accompanied with severe complications.

16 Discussion The second case experienced a relapse event which made it necessary to take HSCT into account very seriously. As there was no donor source available, we had to continue immunosuppressive therapy with steroid and cyclosporine. This will remarkably diminish T- cell function and increase the risk of opportunistic infections. Given that the patient was hypo-gammaglobulinemic as well, the resultant combined immunodeficiency will worsen the issue of recurrent infection

17 Discussion Alternative treatments in non-transplant setting face major limitations Rarity of disease and rarity of clinical trials Limitations of using new drugs for pediatric patients Economic issues Availability of new drugs Higher risk of relapse in pediatric patients due to more infections

18 Other treatment options Combined ATG, corticosteroids, cyclosporine, and intrathecal methotrexate. Combined cyclophosphamide, vincristine, and prednisone. Cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP).

19 Refractory or recurrent disease Alemtuzumab monoclonal antibody to the CD 52 protein expressed on the surface of mature T cells and possibly NK cells. Increased risk of CMV and Adenovirus viremia Use of alemtuzumab before conditioning favorably impacts donor chimerism and is becoming standard peritransplant therapy.

20 Use of alternative donor sources Given the familial clustering of HLH in pediatric cases, unrelated, mismatched, haploidentical, and umbilical cord donors have always been the main stem cell sources for HSCT. In the HLH-94 study, among 124 HSCT recipients, 5 -year survival rates were: 74% for MRD, 76% for MUD, 61% for mismatched unrelated donors (MMUD), 43% for haploidentical donors, 80% for the 10 umbilical cord recipients.

21 Autologous HSCT was considered to be applicable to some subtypes of HLH, such as lymphoma and s. JIA. Combine auto-HSCT with gene therapy to correct the genetic defect. Transfer of a functional perforin gene into autologous hematopoietic stem cells from perforin deficient mice restored perforin expression, partially repaired the cytotoxic defect, and improved HLH symptoms. It will be a great progress in HLH therapy, if this approach can be successfully replicated in human. Carmo M, Risma KA, Arumugam P, et al. Perforin gene transfer into hematopoietic stem cells improves immune dysregulation in murine models of perforin deficiency. Mol Ther 2014; 23: 737– 745.

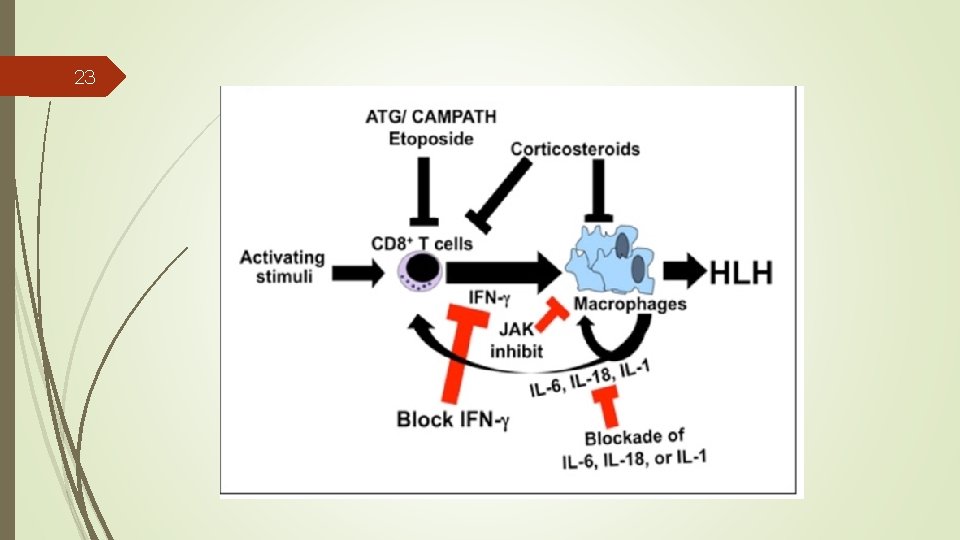
22 Targeted Therapy The chemotherapeutic agents used in conventional treatments have significant toxicities. The limitations of conventional regimens for the treatment of HLH, Increased understanding of key inflammatory molecules.

23

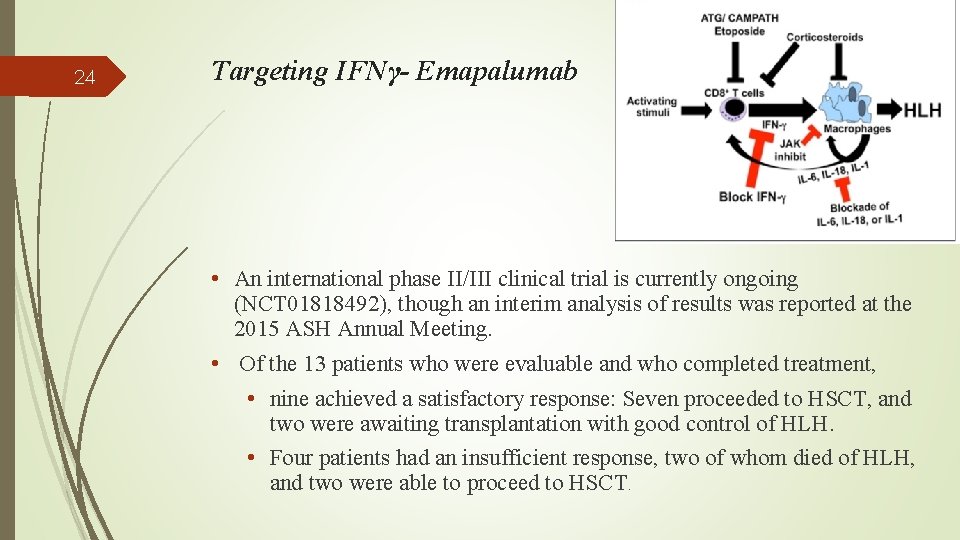
24 Targeting IFNγ- Emapalumab • An international phase II/III clinical trial is currently ongoing (NCT 01818492), though an interim analysis of results was reported at the 2015 ASH Annual Meeting. • Of the 13 patients who were evaluable and who completed treatment, • nine achieved a satisfactory response: Seven proceeded to HSCT, and two were awaiting transplantation with good control of HLH. • Four patients had an insufficient response, two of whom died of HLH, and two were able to proceed to HSCT.

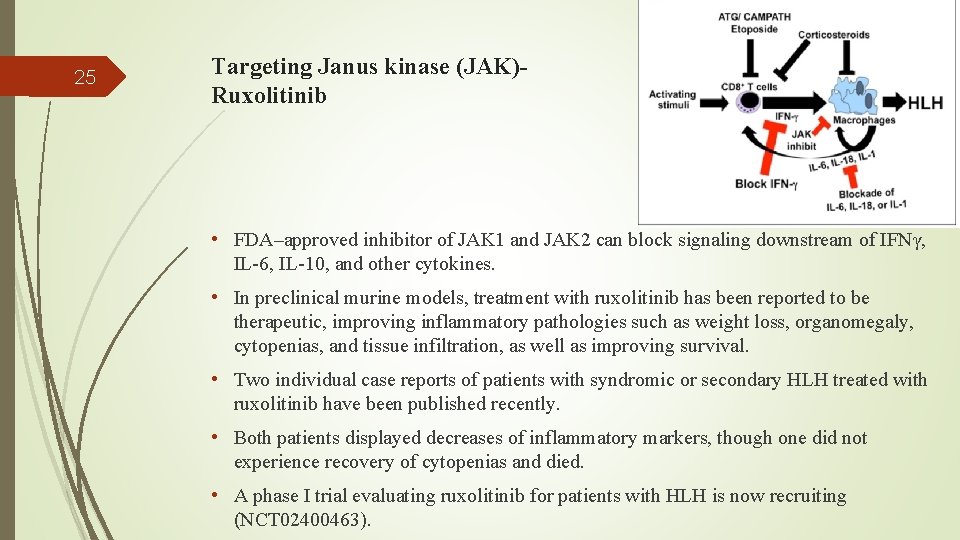
25 Targeting Janus kinase (JAK)Ruxolitinib • FDA–approved inhibitor of JAK 1 and JAK 2 can block signaling downstream of IFNγ, IL-6, IL-10, and other cytokines. • In preclinical murine models, treatment with ruxolitinib has been reported to be therapeutic, improving inflammatory pathologies such as weight loss, organomegaly, cytopenias, and tissue infiltration, as well as improving survival. • Two individual case reports of patients with syndromic or secondary HLH treated with ruxolitinib have been published recently. • Both patients displayed decreases of inflammatory markers, though one did not experience recovery of cytopenias and died. • A phase I trial evaluating ruxolitinib for patients with HLH is now recruiting (NCT 02400463).

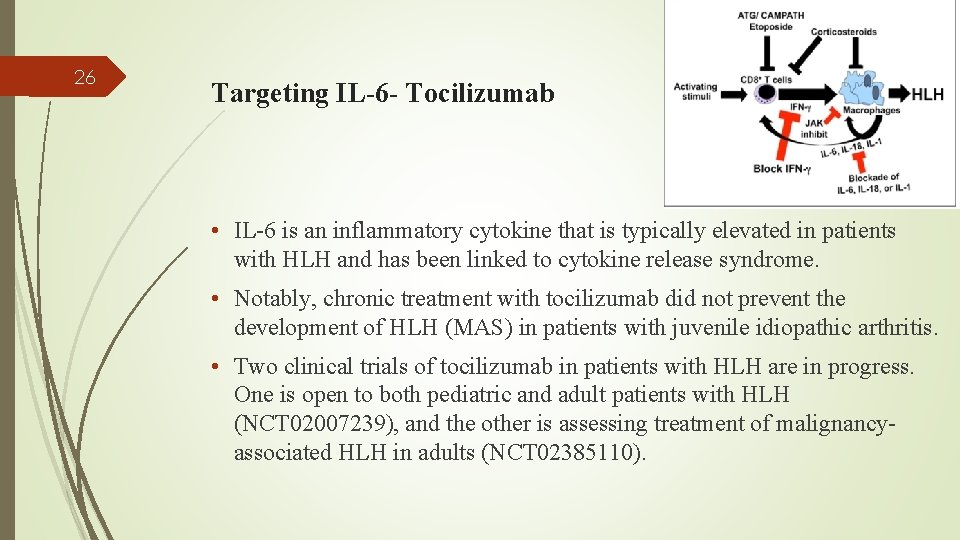
26 Targeting IL-6 - Tocilizumab • IL-6 is an inflammatory cytokine that is typically elevated in patients with HLH and has been linked to cytokine release syndrome. • Notably, chronic treatment with tocilizumab did not prevent the development of HLH (MAS) in patients with juvenile idiopathic arthritis. • Two clinical trials of tocilizumab in patients with HLH are in progress. One is open to both pediatric and adult patients with HLH (NCT 02007239), and the other is assessing treatment of malignancyassociated HLH in adults (NCT 02385110).

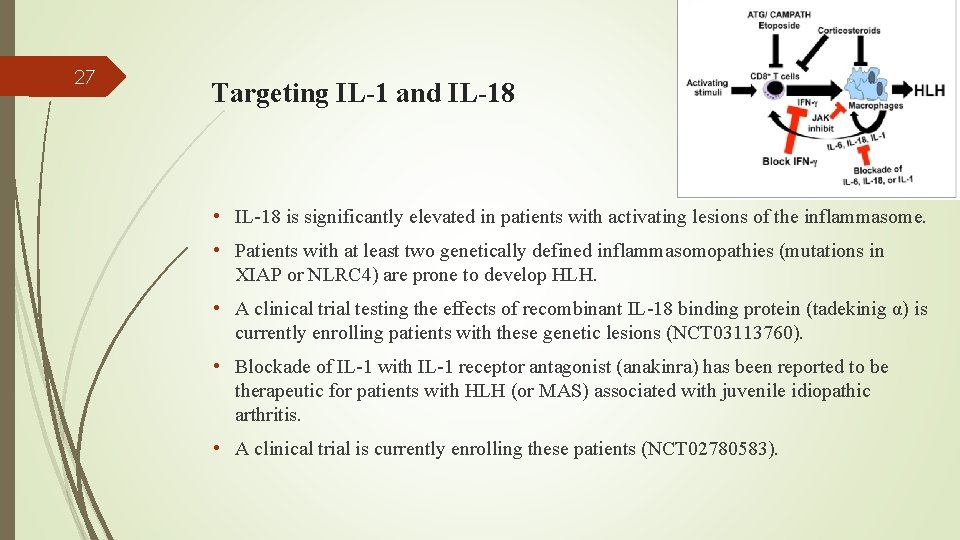
27 Targeting IL-1 and IL-18 • IL-18 is significantly elevated in patients with activating lesions of the inflammasome. • Patients with at least two genetically defined inflammasomopathies (mutations in XIAP or NLRC 4) are prone to develop HLH. • A clinical trial testing the effects of recombinant IL-18 binding protein (tadekinig α) is currently enrolling patients with these genetic lesions (NCT 03113760). • Blockade of IL-1 with IL-1 receptor antagonist (anakinra) has been reported to be therapeutic for patients with HLH (or MAS) associated with juvenile idiopathic arthritis. • A clinical trial is currently enrolling these patients (NCT 02780583).

28 Conclusion HSCT is the treatment of choice in familial HLH as well as recurrent disease and CNS involvement. Finding an HLA-matched donor is the most important obstacle. Alternative treatments in non-transplant setting face major limitations and raise many unresolved questions yet to be answered.

29 As discussed previous: We have two cases of HLH, one with CNS involvement another with relapse No donor sourse available

30 The Best Treatment ? Continue maintenance therapy after 40 weeks ? Stop maintenance therapy after 40 weeks and wait for relapse or not ? Select miss matched unrelated donor ? Try new drugs ?

31 comment Thanks For Your Attention comment commen t
 Understanding jim crow (setting the setting)
Understanding jim crow (setting the setting) Status progress report
Status progress report Sperling letter array experiment
Sperling letter array experiment Two nonadjacent angles formed by two intersecting lines
Two nonadjacent angles formed by two intersecting lines Two little feet
Two little feet Osha two in two out
Osha two in two out Two + two four cryptarithmetic solution
Two + two four cryptarithmetic solution Dentify a key term used in both passages.
Dentify a key term used in both passages. Unit 1 angle relationships
Unit 1 angle relationships When two curves coincide the two objects have the same
When two curves coincide the two objects have the same Identify a key term used in both passages
Identify a key term used in both passages Vertical angles
Vertical angles Two beer or not two beer shakesbeer
Two beer or not two beer shakesbeer Hamlet act 2 scene 1
Hamlet act 2 scene 1 Alan alexander milne
Alan alexander milne Two by two table
Two by two table Shall i compare thee to a summer's day annotation
Shall i compare thee to a summer's day annotation Which witch watches which watch
Which witch watches which watch Two beer or not two beer shakesbeer
Two beer or not two beer shakesbeer What kind of person is malcolm in macbeth
What kind of person is malcolm in macbeth When mr pirzada came to dine character analysis
When mr pirzada came to dine character analysis Ball setting and streaming
Ball setting and streaming What is setting and plot
What is setting and plot Dts gate motor pc board price
Dts gate motor pc board price Gregory bateson framing theory
Gregory bateson framing theory Charlie and the chocolate factory house
Charlie and the chocolate factory house Characters of transforming moments
Characters of transforming moments Iec 60076 power transformers
Iec 60076 power transformers Thank you ma'am setting
Thank you ma'am setting Setting of to kill a mockingbird
Setting of to kill a mockingbird Chapter 1 of to kill a mockingbird
Chapter 1 of to kill a mockingbird Chapter 6 to kill a mockingbird
Chapter 6 to kill a mockingbird