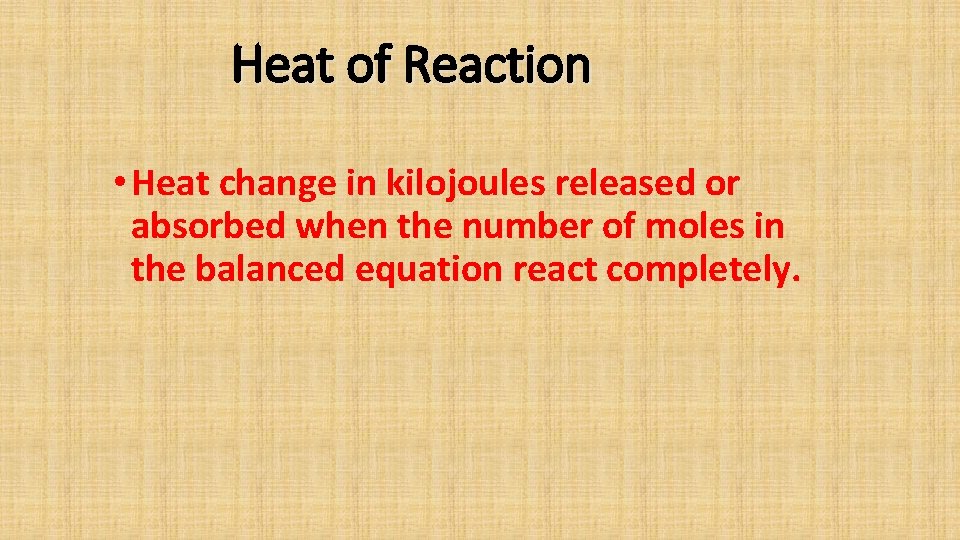
Heat of Reaction Heat change in kilojoules released
![Heat of Reaction [i] H 2(g) + 1/2 O 2(g) = H 2 O(g) Heat of Reaction [i] H 2(g) + 1/2 O 2(g) = H 2 O(g)](https://slidetodoc.com/presentation_image_h2/afaa7beb553c7bc4fe34283064fdc690/image-3.jpg)







- Slides: 17

Heat of Reaction • Heat change in kilojoules released or absorbed when the number of moles in the balanced equation react completely.

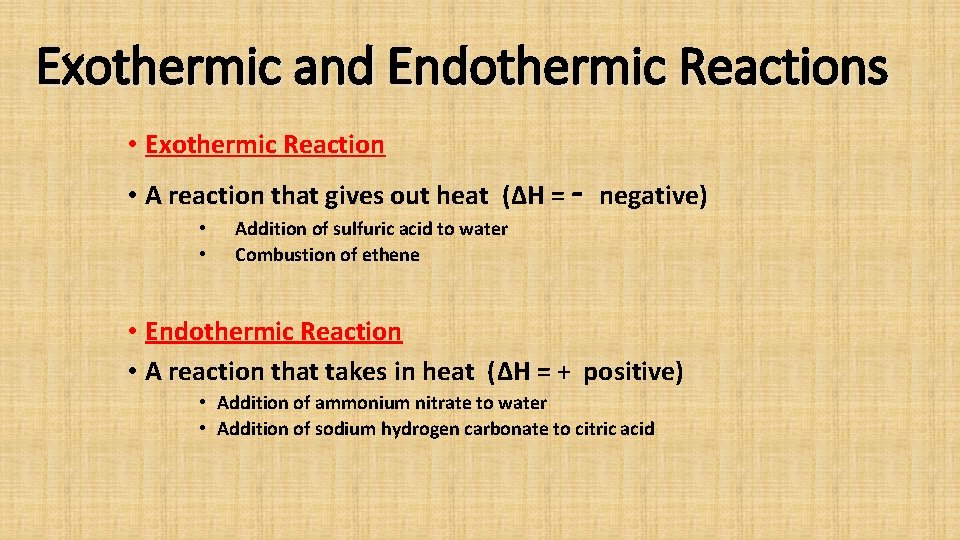
Exothermic and Endothermic Reactions • Exothermic Reaction • A reaction that gives out heat (ΔH = - negative) • • Addition of sulfuric acid to water Combustion of ethene • Endothermic Reaction • A reaction that takes in heat (ΔH = + positive) • Addition of ammonium nitrate to water • Addition of sodium hydrogen carbonate to citric acid
![Heat of Reaction i H 2g 12 O 2g H 2 Og Heat of Reaction [i] H 2(g) + 1/2 O 2(g) = H 2 O(g)](https://slidetodoc.com/presentation_image_h2/afaa7beb553c7bc4fe34283064fdc690/image-3.jpg)
Heat of Reaction [i] H 2(g) + 1/2 O 2(g) = H 2 O(g) H = -242 k. J [ii] 2 H 2(g) + O 2(g) = 2 H 2 O(g) H = -484 k. J [iii] H 2(g) + 1/2 O 2(g) = H 2 O(l) H = -282 k. J What is the difference between the above reactions?

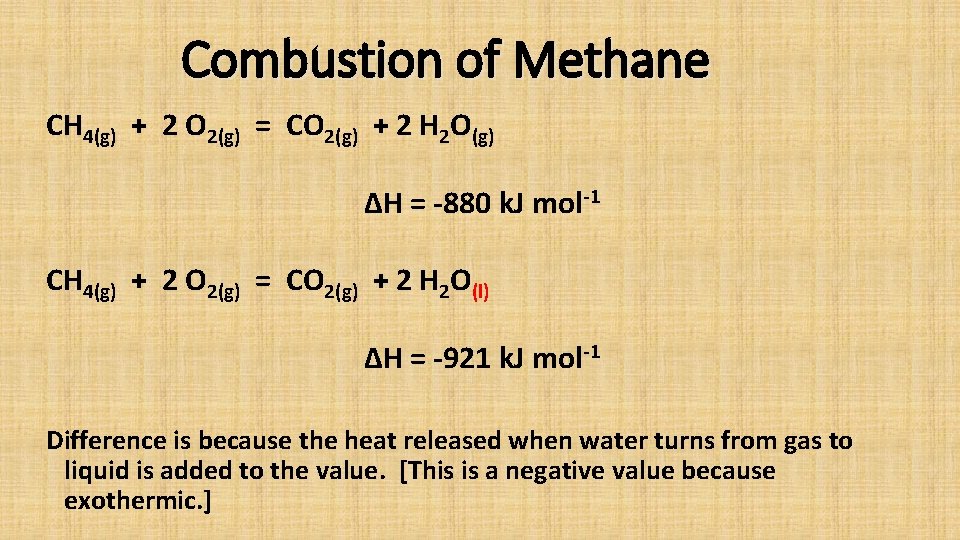
Combustion of Methane CH 4(g) + 2 O 2(g) = CO 2(g) + 2 H 2 O(g) ΔH = -880 k. J mol-1 CH 4(g) + 2 O 2(g) = CO 2(g) + 2 H 2 O(l) ΔH = -921 k. J mol-1 Difference is because the heat released when water turns from gas to liquid is added to the value. [This is a negative value because exothermic. ]

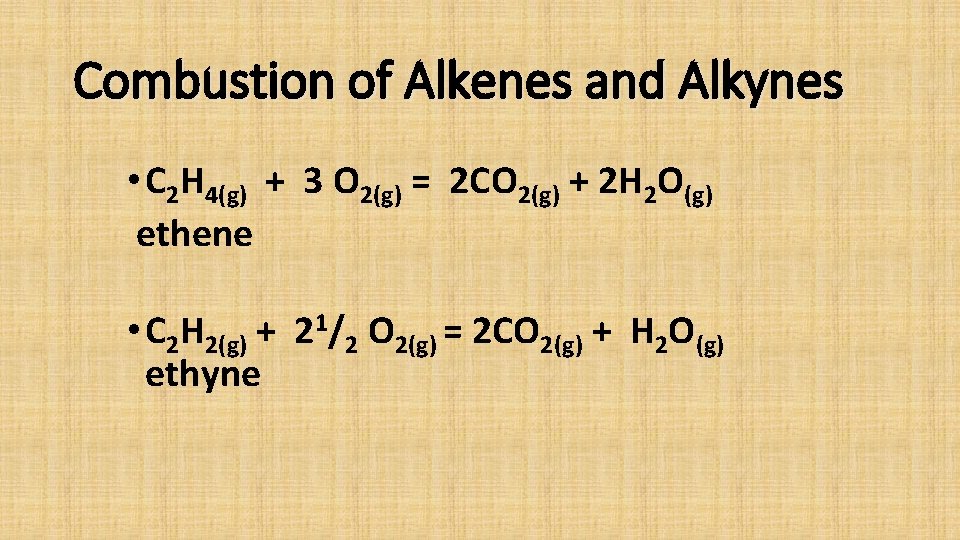
Combustion of Alkenes and Alkynes • C 2 H 4(g) + 3 O 2(g) = 2 CO 2(g) + 2 H 2 O(g) ethene • C 2 H 2(g) + 21/2 O 2(g) = 2 CO 2(g) + H 2 O(g) ethyne

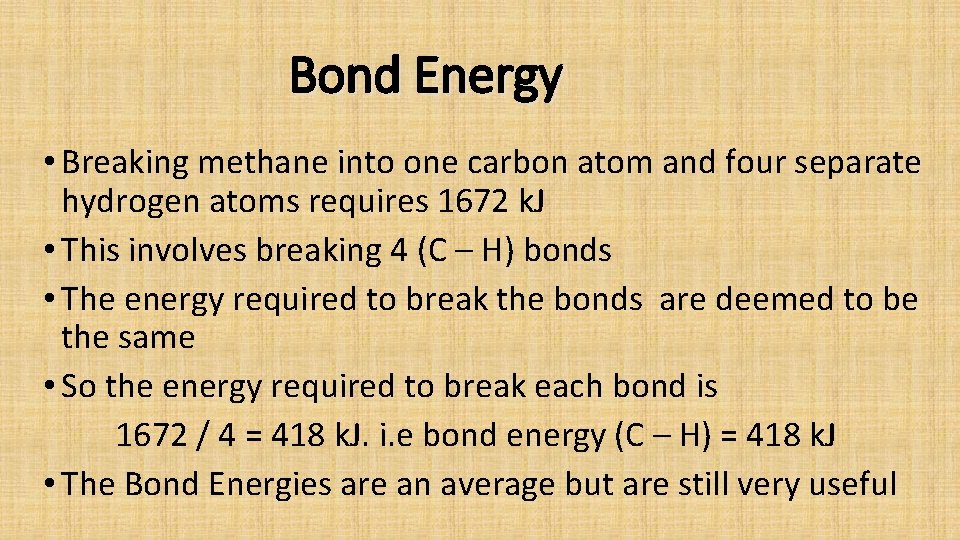
Bond Energy Amount of energy in k. J required to break one mole of covalent bonds and to separate the atoms completely from each other.

Bond Energy • CH 4 + 2 O 2 = CO 2 + 2 H 2 O • What happens to the CH 4 and O 2 in the reaction? • Bonds must first be broken into their separate atoms and then reassembled. • To understand what is happening we must look at their structure in detail.


Bond Energy • Breaking methane into one carbon atom and four separate hydrogen atoms requires 1672 k. J • This involves breaking 4 (C – H) bonds • The energy required to break the bonds are deemed to be the same • So the energy required to break each bond is 1672 / 4 = 418 k. J. i. e bond energy (C – H) = 418 k. J • The Bond Energies are an average but are still very useful

Heat of Combustion Heat change when one mole of a substance is completely burned in excess oxygen SCC Science Dept

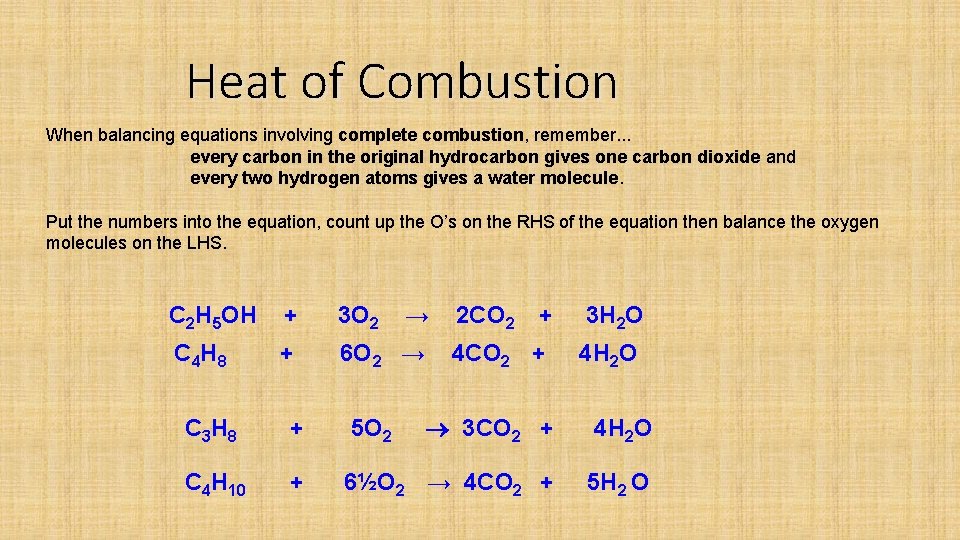
Heat of Combustion When balancing equations involving complete combustion, remember. . . every carbon in the original hydrocarbon gives one carbon dioxide and every two hydrogen atoms gives a water molecule. Put the numbers into the equation, count up the O’s on the RHS of the equation then balance the oxygen molecules on the LHS. C 2 H 5 OH + 3 O 2 → C 4 H 8 + 6 O 2 → 2 CO 2 + 3 H 2 O 4 CO 2 + 4 H 2 O C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O C 4 H 10 + 6½O 2 → 4 CO 2 + 5 H 2 O

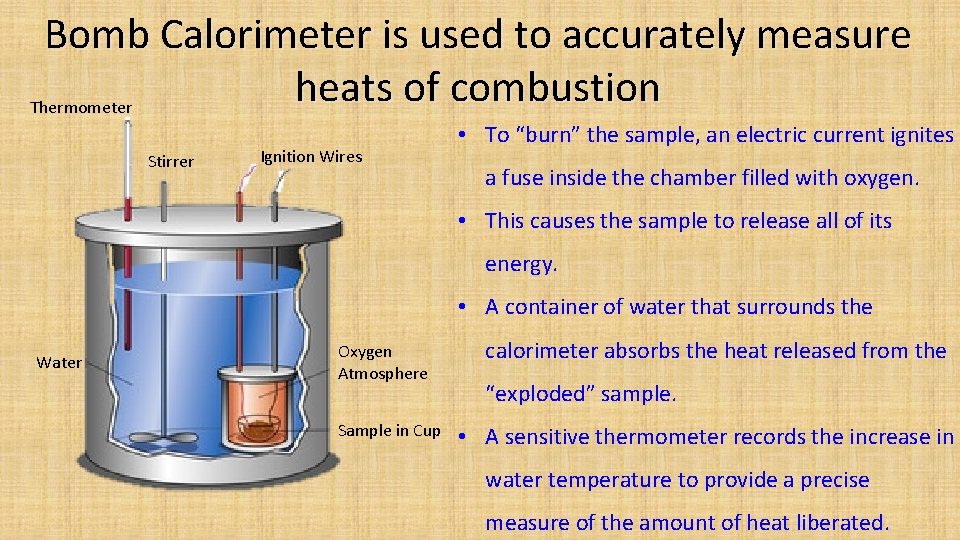
Bomb Calorimeter is used to accurately measure heats of combustion Thermometer Stirrer Ignition Wires • To “burn” the sample, an electric current ignites a fuse inside the chamber filled with oxygen. • This causes the sample to release all of its energy. • A container of water that surrounds the Water Oxygen Atmosphere Sample in Cup calorimeter absorbs the heat released from the “exploded” sample. • A sensitive thermometer records the increase in water temperature to provide a precise measure of the amount of heat liberated.

Uses of Bomb Calorimeter • To determine heats of combustion of different fuels • Bomb Calorimeter can determine the calorific values of food • Used to compare the calorific (energy) values of fuels • Used to find the energy value of a fuel

Kilogram Calorific Value in k. J per kg • Heat produced in k. J when 1 kg of a substance is completely burned in excess oxygen • Coal 25000 - 33000 • Oil 45000 • Paraffin 48000 • Ethanol 30000 • Natural Gas - 55000 • Hydrogen - 143000

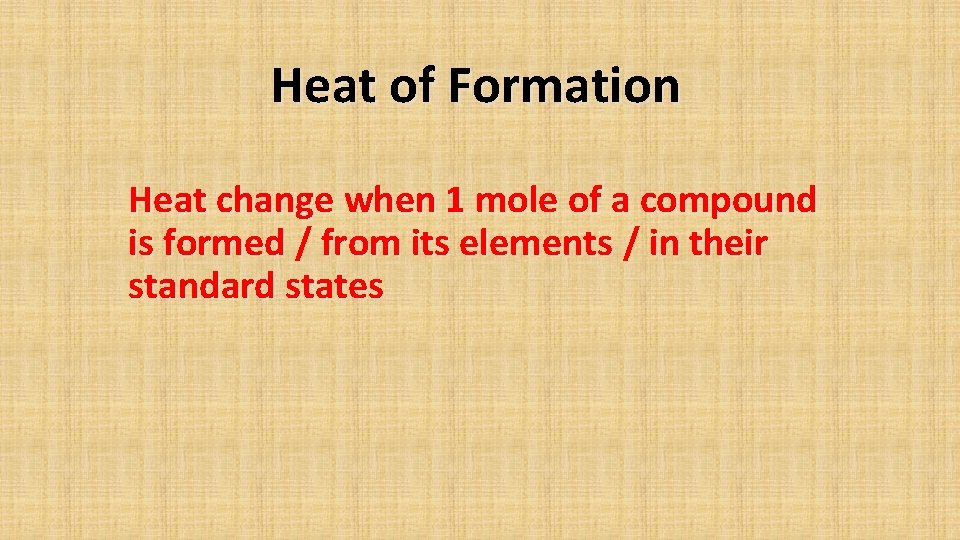
Heat of Formation Heat change when 1 mole of a compound is formed / from its elements / in their standard states

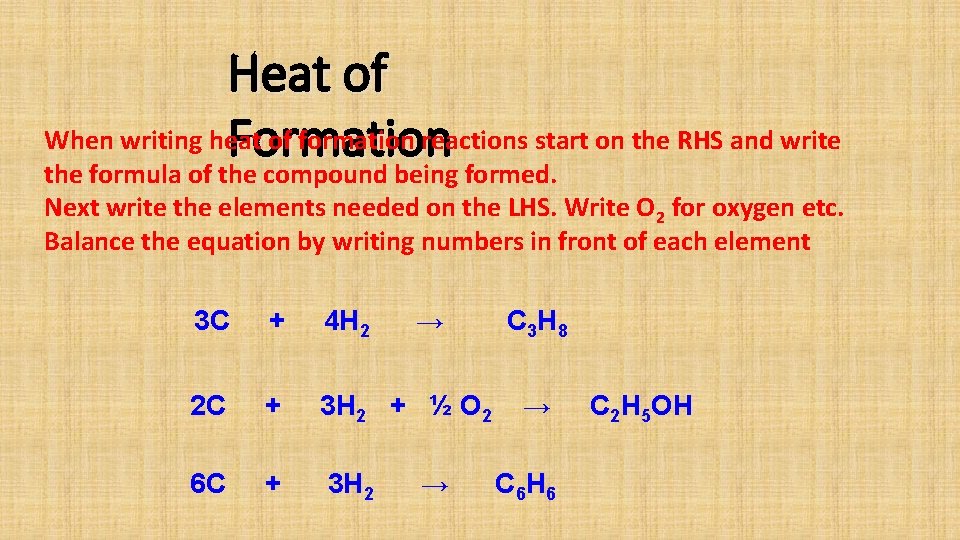
Heat of When writing heat of formation reactions start on the RHS and write Formation the formula of the compound being formed. Next write the elements needed on the LHS. Write O 2 for oxygen etc. Balance the equation by writing numbers in front of each element 3 C + 4 H 2 → 2 C + 3 H 2 + ½ O 2 6 C + 3 H 2 → C 3 H 8 → C 6 H 6 C 2 H 5 OH

Law of Conservation of Energy is neither created nor destroyed but simply changes from one form to another.
 Specific latent heat of a substance
Specific latent heat of a substance Heat released
Heat released Unit of rate of reaction for first order reaction
Unit of rate of reaction for first order reaction Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Half life formula
Half life formula Enthalpy calculation
Enthalpy calculation Enthalpy change definition
Enthalpy change definition Energetically stable meaning
Energetically stable meaning How to balance a chemical equation
How to balance a chemical equation Is the halloween clock reaction a chemical change
Is the halloween clock reaction a chemical change Odor change chemical reaction example
Odor change chemical reaction example Staar released test
Staar released test Staar released test
Staar released test Staar alt 2
Staar alt 2 Ricas released
Ricas released Released rta passages
Released rta passages Adh released from
Adh released from