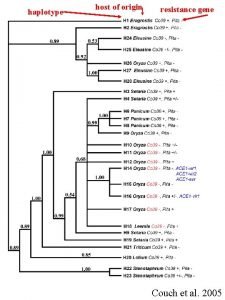
haplotype host of origin resistance gene Couch et

- Slides: 19

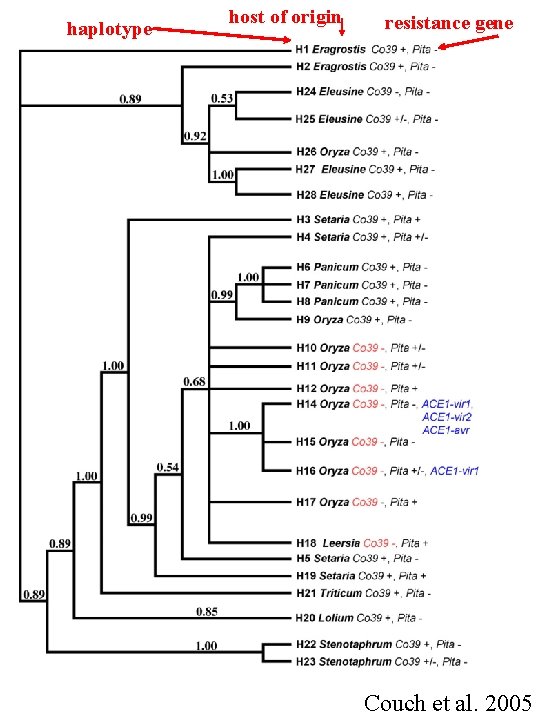
haplotype host of origin resistance gene Couch et al. 2005

Magnaporthe oryzae

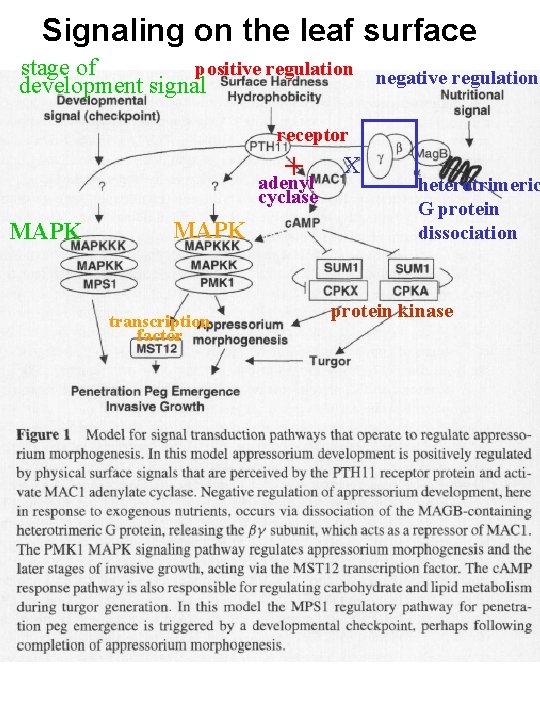
Signaling on the leaf surface positive regulation negative regulation stage of development signal receptor + adenyl cyclase MAPK transcription factor X heterotrimeric G protein dissociation protein kinase

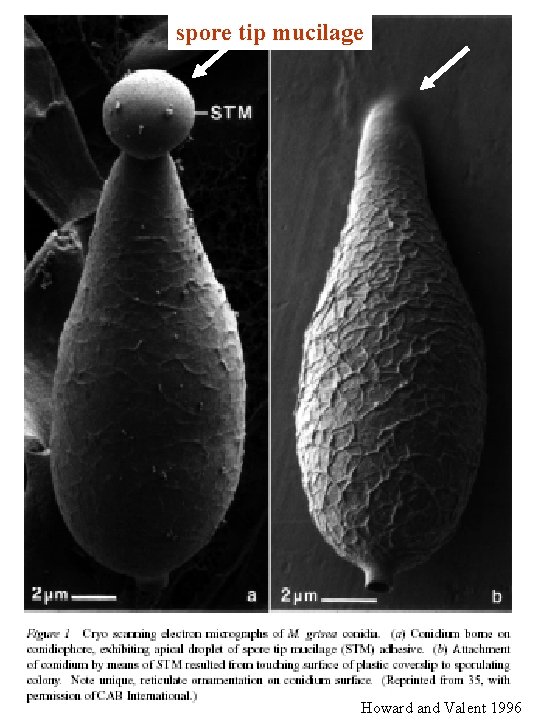
spore tip mucilage Howard and Valent 1996

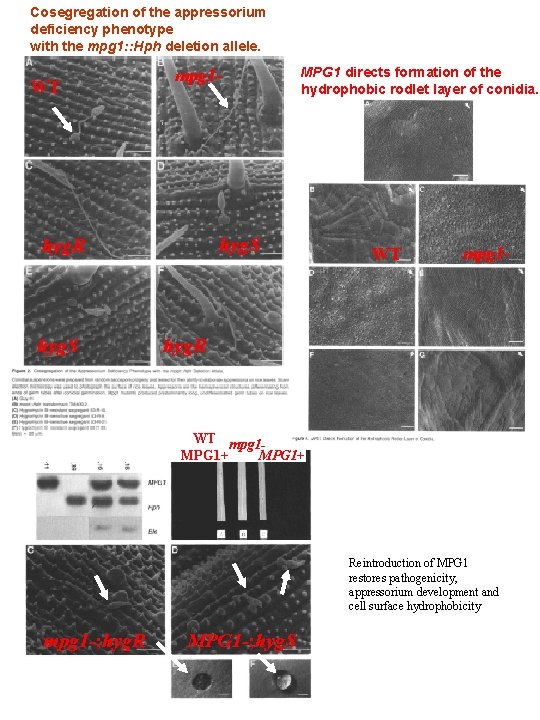
Cosegregation of the appressorium deficiency phenotype with the mpg 1: : Hph deletion allele. WT mpg 1 - hyg. S hyg. R hyg. S MPG 1 directs formation of the hydrophobic rodlet layer of conidia. WT mpg 1 - hyg. R WT mpg 1 MPG 1+ Reintroduction of MPG 1 restores pathogenicity, appressorium development and cell surface hydrophobicity mpg 1 -; hyg. R MPG 1 -; hyg. S

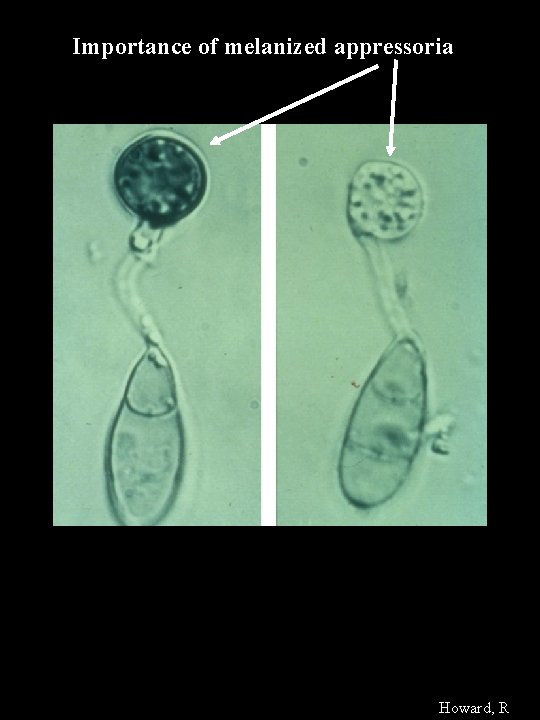
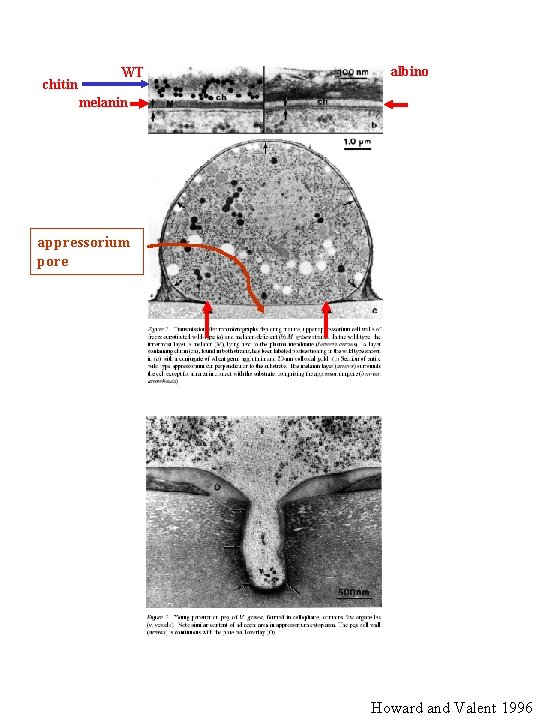
Importance of melanized appressoria Howard, R

chitin WT albino melanin appressorium pore Howard and Valent 1996

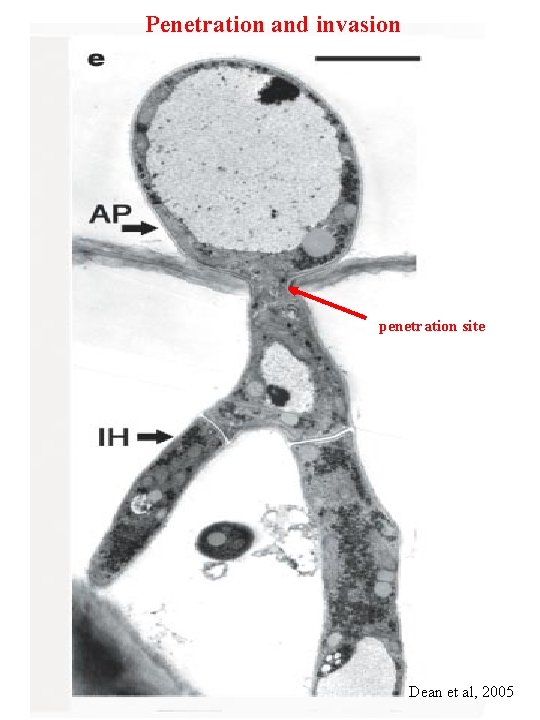
Penetration and invasion penetration site Dean et al, 2005

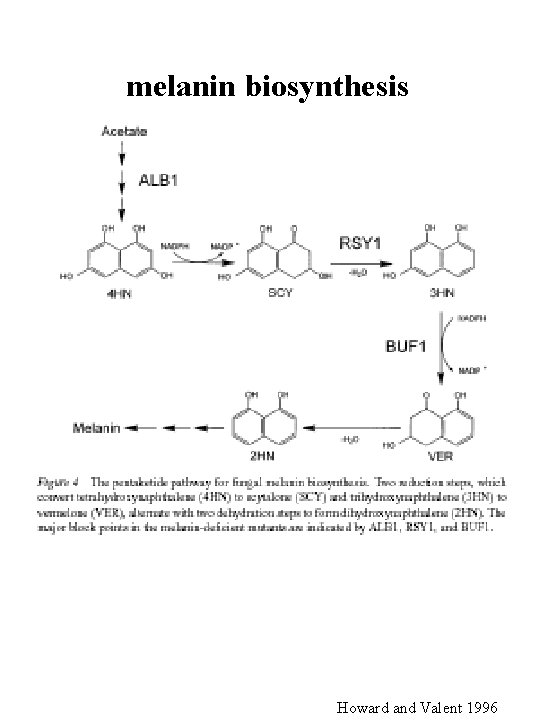
melanin biosynthesis Howard and Valent 1996

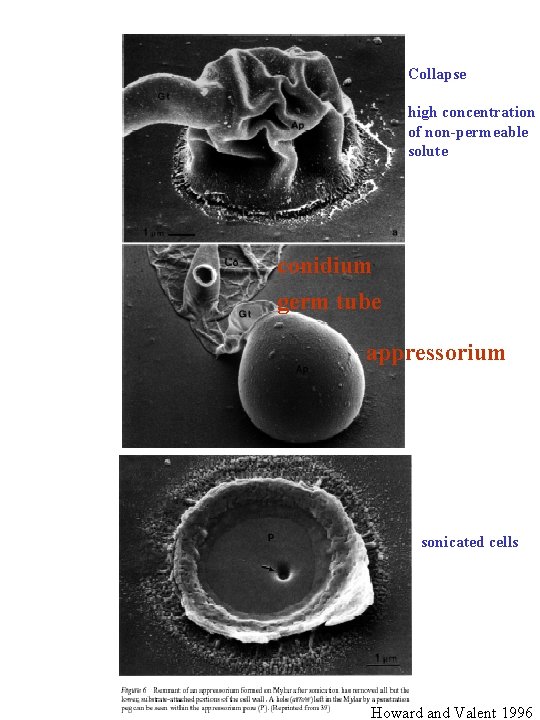
Collapse high concentration of non-permeable solute conidium germ tube appressorium sonicated cells Howard and Valent 1996

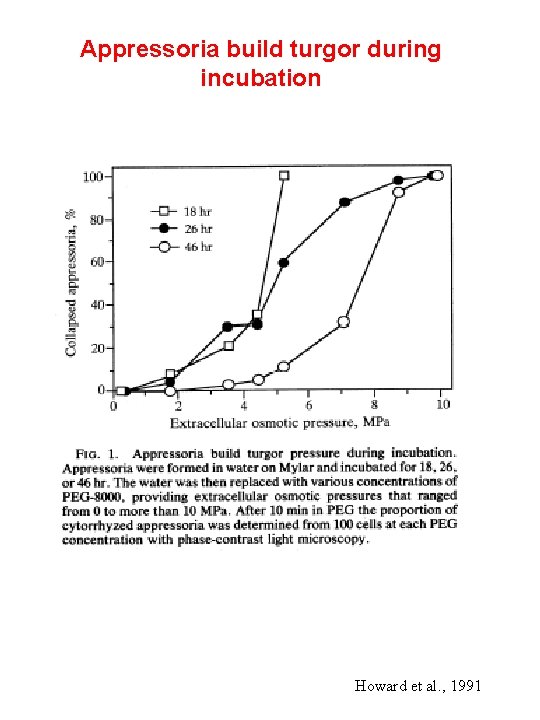
Appressoria build turgor during incubation Howard et al. , 1991

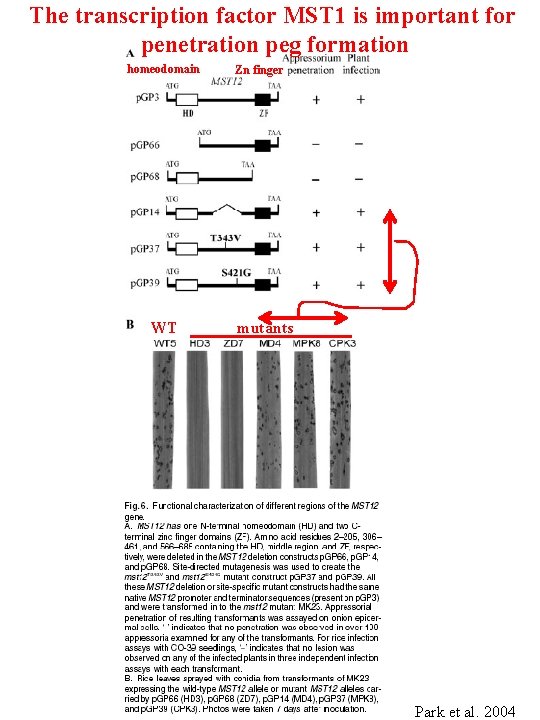
The transcription factor MST 1 is important for penetration peg formation homeodomain WT Zn finger mutants Park et al. 2004

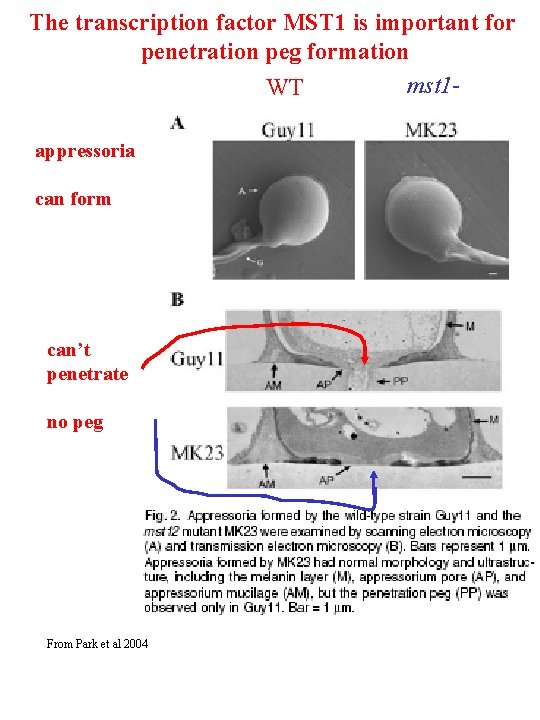
The transcription factor MST 1 is important for penetration peg formation mst 1 WT appressoria can form can’t penetrate no peg From Park et al 2004

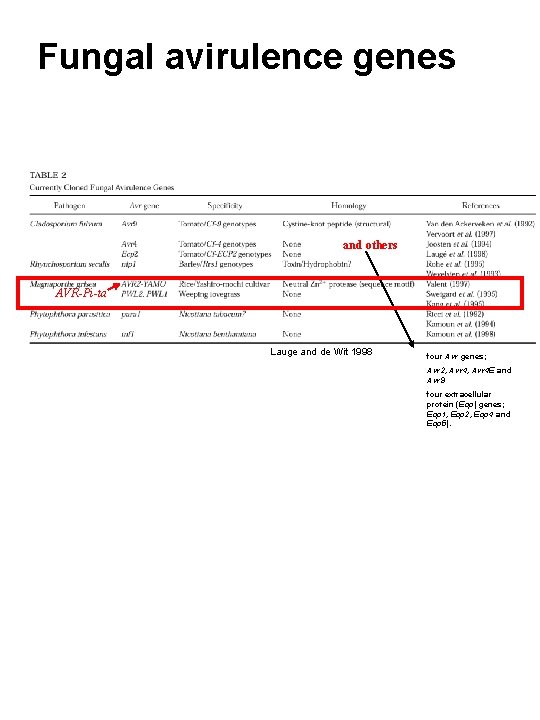
Fungal avirulence genes and others AVR-Pi-ta Lauge and de Wit 1998 four Avr genes; Avr 2, Avr 4 E and Avr 9 four extracellular protein (Ecp) genes; Ecp 1, Ecp 2, Ecp 4 and Ecp 5).

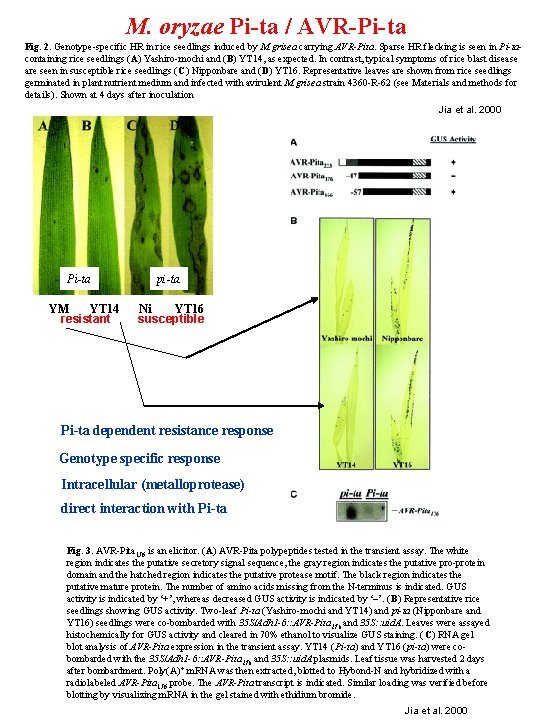
M. oryzae Pi-ta / AVR-Pi-ta Fig. 2. Genotype-specific HR in rice seedlings induced by M. grisea carrying AVR-Pita. Sparse HR flecking is seen in Pi-tacontaining rice seedlings (A) Yashiro-mochi and (B) YT 14, as expected. In contrast, typical symptoms of rice blast disease are seen in susceptible rice seedlings (C) Nipponbare and (D) YT 16. Representative leaves are shown from rice seedlings germinated in plant nutrient medium and infected with avirulent M. grisea strain 4360 -R-62 (see Materials and methods for details). Shown at 4 days after inoculation Jia et al. 2000 Pi-ta YM YT 14 resistant pi-ta Ni YT 16 susceptible Pi-ta dependent resistance response Genotype specific response Intracellular (metalloprotease) direct interaction with Pi-ta Fig. 3. AVR-Pita 176 is an elicitor. (A) AVR-Pita polypeptides tested in the transient assay. The white region indicates the putative secretory signal sequence, the gray region indicates the putative pro-protein domain and the hatched region indicates the putative protease motif. The black region indicates the putative mature protein. The number of amino acids missing from the N-terminus is indicated. GUS activity is indicated by ‘+’, whereas decreased GUS activity is indicated by ‘–’. ( B) Representative rice seedlings showing GUS activity. Two-leaf Pi-ta (Yashiro-mochi and YT 14) and pi-ta (Nipponbare and YT 16) seedlings were co-bombarded with 35 S/Adh 1 -6: : AVR-Pita 176 and 35 S: : uid. A. Leaves were assayed histochemically for GUS activity and cleared in 70% ethanol to visualize GUS staining. (C) RNA gel blot analysis of AVR-Pita expression in the transient assay. YT 14 (Pi-ta) and YT 16 (pi-ta) were cobombarded with the 35 S/Adh 1 -6: : AVR-Pita 176 and 35 S: : uid. A plasmids. Leaf tissue was harvested 2 days after bombardment. Poly(A) + m. RNA was then extracted, blotted to Hybond-N and hybridized with a radiolabeled AVR-Pita 176 probe. The AVR-Pita transcript is indicated. Similar loading was verified before blotting by visualizing m. RNA in the gel stained with ethidium bromide. Jia et al. 2000

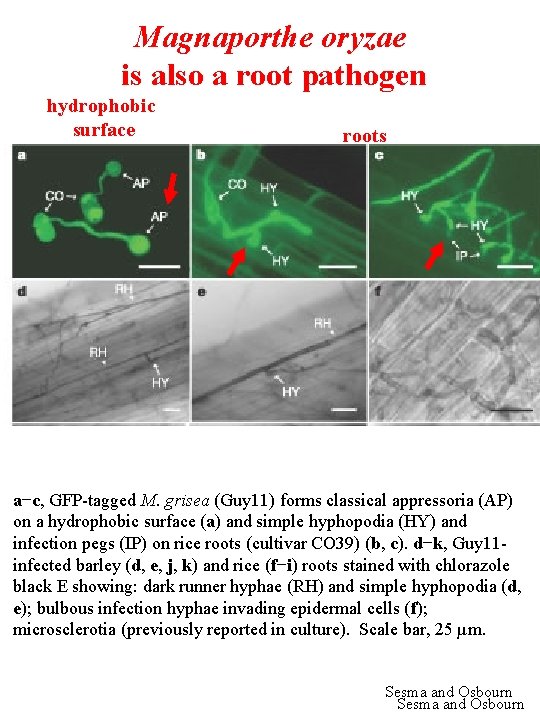
Magnaporthe oryzae is also a root pathogen hydrophobic surface roots a−c, GFP-tagged M. grisea (Guy 11) forms classical appressoria (AP) on a hydrophobic surface (a) and simple hyphopodia (HY) and infection pegs (IP) on rice roots (cultivar CO 39) (b, c). d−k, Guy 11 infected barley (d, e, j, k) and rice (f−i) roots stained with chlorazole black E showing: dark runner hyphae (RH) and simple hyphopodia (d, e); bulbous infection hyphae invading epidermal cells (f); microsclerotia (previously reported in culture). Scale bar, 25 µm. Sesma and Osbourn

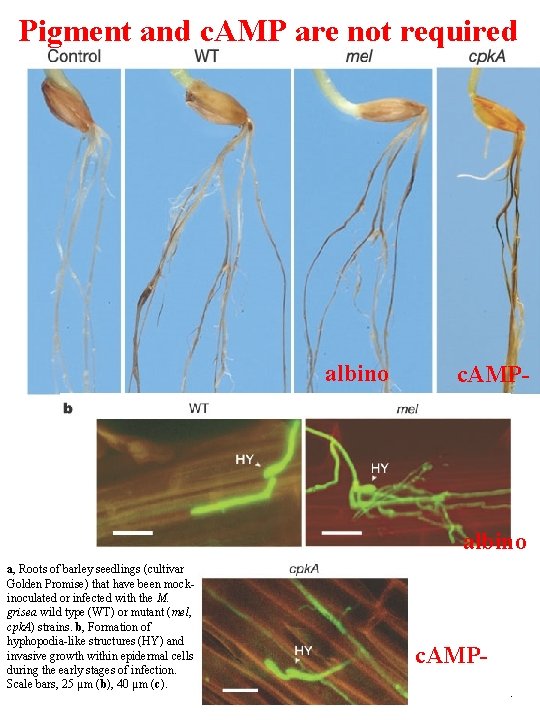
Pigment and c. AMP are not required albino c. AMP- albino a, Roots of barley seedlings (cultivar Golden Promise) that have been mockinoculated or infected with the M. grisea wild type (WT) or mutant (mel, cpk. A) strains. b, Formation of hyphopodia-like structures (HY) and invasive growth within epidermal cells during the early stages of infection. Scale bars, 25 µm (b), 40 µm (c). c. AMPSesma and Osbourn

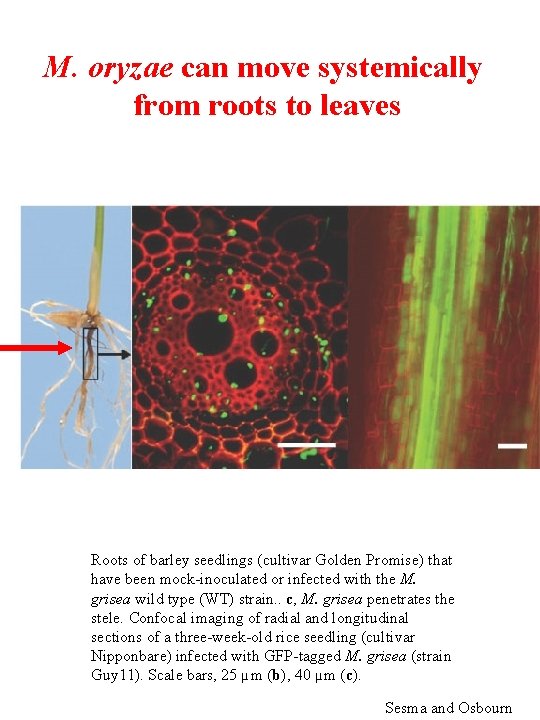
M. oryzae can move systemically from roots to leaves Roots of barley seedlings (cultivar Golden Promise) that have been mock-inoculated or infected with the M. grisea wild type (WT) strain. . c, M. grisea penetrates the stele. Confocal imaging of radial and longitudinal sections of a three-week-old rice seedling (cultivar Nipponbare) infected with GFP-tagged M. grisea (strain Guy 11). Scale bars, 25 µm (b), 40 µm (c). Sesma and Osbourn

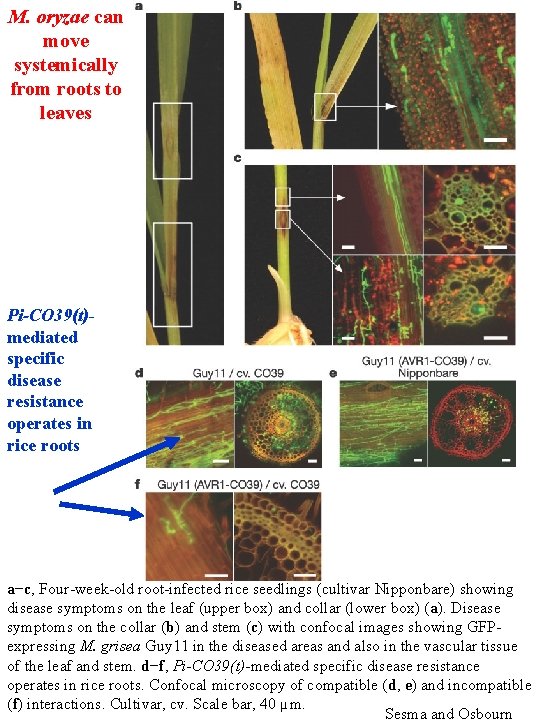
M. oryzae can move systemically from roots to leaves Pi-CO 39(t)mediated specific disease resistance operates in rice roots a−c, Four-week-old root-infected rice seedlings (cultivar Nipponbare) showing disease symptoms on the leaf (upper box) and collar (lower box) (a). Disease symptoms on the collar (b) and stem (c) with confocal images showing GFPexpressing M. grisea Guy 11 in the diseased areas and also in the vascular tissue of the leaf and stem. d−f, Pi-CO 39(t)-mediated specific disease resistance operates in rice roots. Confocal microscopy of compatible (d, e) and incompatible (f) interactions. Cultivar, cv. Scale bar, 40 µm. Sesma and Osbourn
 Definitive host vs intermediate host
Definitive host vs intermediate host Haplotype
Haplotype Haplotype example
Haplotype example Haplotype
Haplotype Haplotype vs genotype
Haplotype vs genotype Haplotype
Haplotype Gene by gene test results
Gene by gene test results Protein power point
Protein power point Specific cake resistance formula
Specific cake resistance formula Ignoring friction air resistance and electrical resistance
Ignoring friction air resistance and electrical resistance Tramp stamp kenning
Tramp stamp kenning How to draw a dresser in one point perspective
How to draw a dresser in one point perspective Finger couch
Finger couch Couchsurfing questions
Couchsurfing questions Couch rodzajnik
Couch rodzajnik Couch potato kenning
Couch potato kenning Stefan couch
Stefan couch Stefan couch
Stefan couch Literal couch potato
Literal couch potato Brian couch attorney hyden ky
Brian couch attorney hyden ky