Genetics made easy demystifying genetic testing DR CATHERINE













- Slides: 20

Genetics made easy: demystifying genetic testing DR CATHERINE MCWILLIAM DAWN O’SULLIVAN

Workshop Aims An overview of the role of clinical and molecular genetics How to arrange genetic testing Interpreting results Case examples

Inherited Cardiac Disease Hypertrophic Cardiomyopathy Familial Arrhythmia Long QT syndrome Brugada Arrhythmogenic right ventricular dysplasia/cardiomyopathy Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) Inherited aortopathies Marfan, Loeys Dietz Dilated Cardiomyopathy Around 50% maybe caused by a dominantly inherited pathogenic variant Fewer genetic causes but worth considering Congenital cardiac disease

Genetic Testing: What are the pro’s and cons?

Genetic Testing: pros and cons Pros Cons To allow identification of at risk relatives Targeted screening Allowing early diagnosis and intervention Confirm diagnosis At risk relatives may not wish to know May not clarify diagnosis if variant of uncertain significance identified Feelings of guilt if passed on to younger generation Predictive testing may turn the ‘well’ individual into a patient Employment/Finance/ Insurance issues



Consent: What do we need to think about?

Consent To a specific test Information to be shared with other family members on request of their doctor Information to be used to allow accurate testing of family members To DNA storage For DNA to be used for validating test (ie within the lab, anonymously) For DNA to be used in research

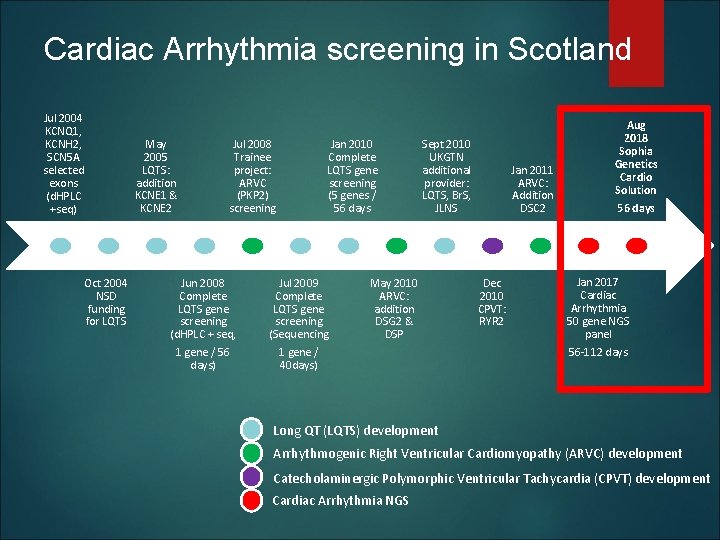
Cardiac Arrhythmia screening in Scotland Jul 2004 KCNQ 1, KCNH 2, SCN 5 A selected exons (d. HPLC +seq) May 2005 LQTS: addition KCNE 1 & KCNE 2 Oct 2004 NSD funding for LQTS Jul 2008 Trainee project: ARVC (PKP 2) screening Jan 2010 Complete LQTS gene screening (5 genes / 56 days Jun 2008 Complete LQTS gene screening (d. HPLC + seq, Jul 2009 Complete LQTS gene screening (Sequencing 1 gene / 56 days) 1 gene / 40 days) Sept 2010 UKGTN additional provider: LQTS, Br. S, JLNS May 2010 ARVC: addition DSG 2 & DSP Jan 2011 ARVC: Addition DSC 2 Dec 2010 CPVT: RYR 2 Aug 2018 Sophia Genetics Cardio Solution 56 days Jan 2017 Cardiac Arrhythmia 50 gene NGS panel 56 -112 days Long QT (LQTS) development Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) development Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) development Cardiac Arrhythmia NGS

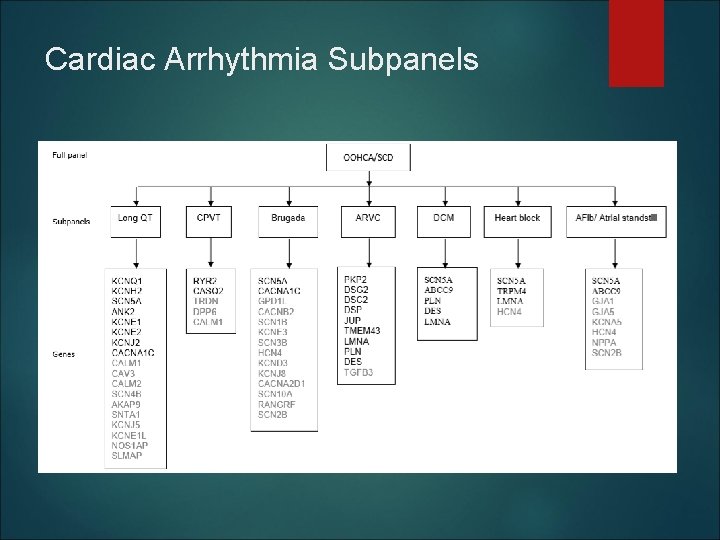
Cardiac Arrhythmia Subpanels

Variant classification Implementation of NGS (bigger panels) results in more Variants of Uncertain Clinical Significance (VUS) Very little evidence available in the literature / databases Guidelines are intended for general use in classifying variants in patients with rare diseases. Disease-specific guidelines are being developed for disorders where different evidence thresholds are required, e. g. cardiac disorders Still framework open to interpretation and need for professional judgement

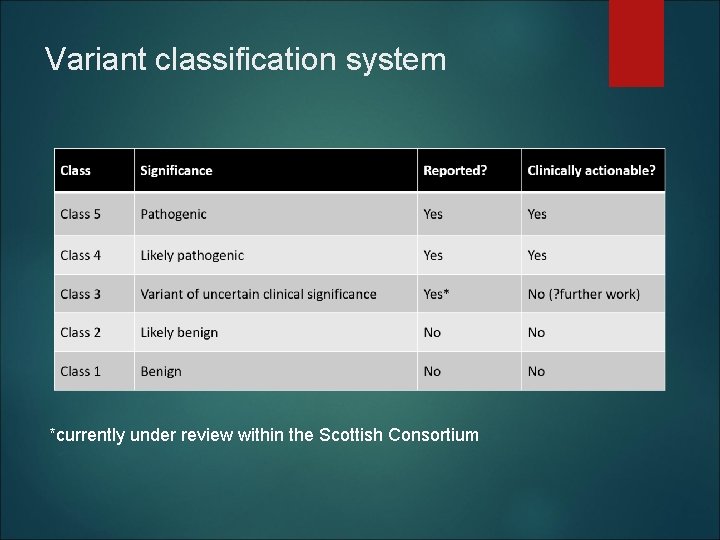
Variant classification system *currently under review within the Scottish Consortium

Variant classification • Rules where clinical input required – PS 2 De novo (both maternity and paternity confirmed) in patient with the disease and no FH – PM 6 Assumed de novo, but without confirmation of paternity and maternity – PP 1 Cosegregation with disease in multiple affected family members in a gene definitively known to cause the disease (may be used as stronger evidence with increasing segregation data) – PP 4 Patient’s phenotype or family history is highly specific for a disease with a single genetic etiology • Greater need for good clinical and laboratory communication – Regular MDTs (lab, clinical genetics, other specialists) / VUS classification meetings – Details in referral important: patient phenotype if known and what clinical tests carried out, specific relationship to the patient, segregation data – This evidence must be formally recorded rather than verbal communication • Likely to be a change in how VUS are reported but communication will still be essential

Cases

Learning point Variant reclassification

Learning point Availability of genetic testing is expanding It can be worth looking again….

Learning point Beware the non-informative family history Complexities of cascade screening in a new diagnosis

Learning point Cascade screening in a timely manner/as suggested by the presentation

Summary Genetic testing can be very helpful to support diagnosis and target screening for family members Molecular genetics is complex and not always easily interpretable – if in doubt please ask! Patients and families can find this complexity difficult and it is better to make them aware of the possibility of ‘variant of unknown significance’ at the start of the journey