Gas Laws Charless Law V 1 x T


- Slides: 9

Gas Laws

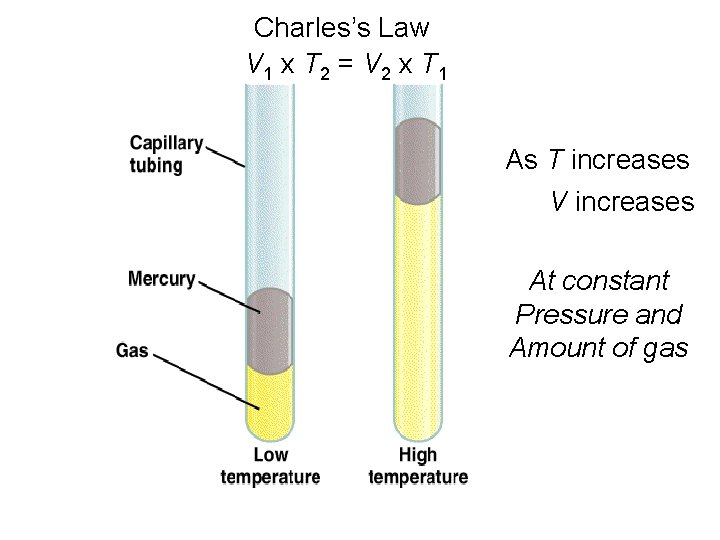
Charles’s Law V 1 x T 2 = V 2 x T 1 As T increases V increases At constant Pressure and Amount of gas

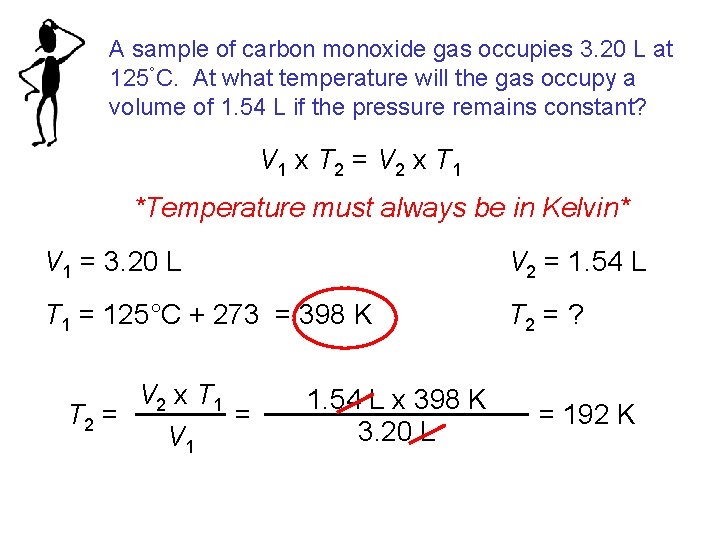
A sample of carbon monoxide gas occupies 3. 20 L at 125°C. At what temperature will the gas occupy a volume of 1. 54 L if the pressure remains constant? V 1 x T 2 = V 2 x T 1 *Temperature must always be in Kelvin* V 1 = 3. 20 L V 2 = 1. 54 L T 1 = 125°C + 273 = 398 K T 2 = ? V 2 x T 1 = T 2 = V 1 1. 54 L x 398 K 3. 20 L = 192 K

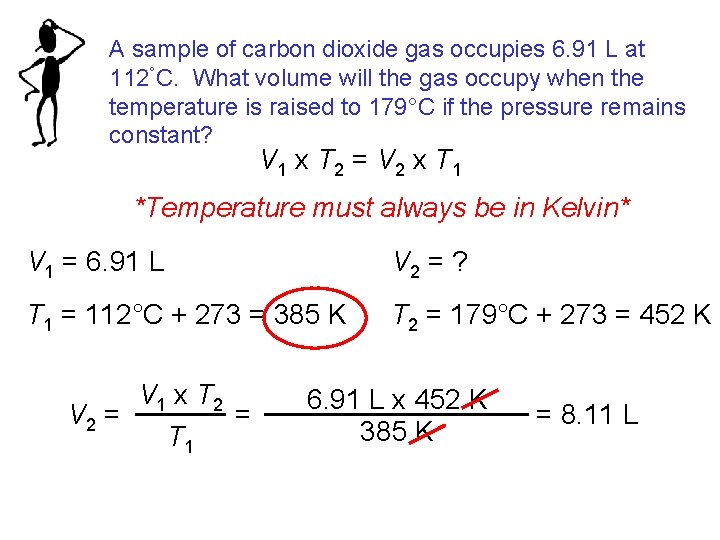
A sample of carbon dioxide gas occupies 6. 91 L at 112°C. What volume will the gas occupy when the temperature is raised to 179°C if the pressure remains constant? V 1 x T 2 = V 2 x T 1 *Temperature must always be in Kelvin* V 1 = 6. 91 L V 2 = ? T 1 = 112°C + 273 = 385 K T 2 = 179°C + 273 = 452 K V 1 x T 2 = V 2 = T 1 6. 91 L x 452 K 385 K = 8. 11 L

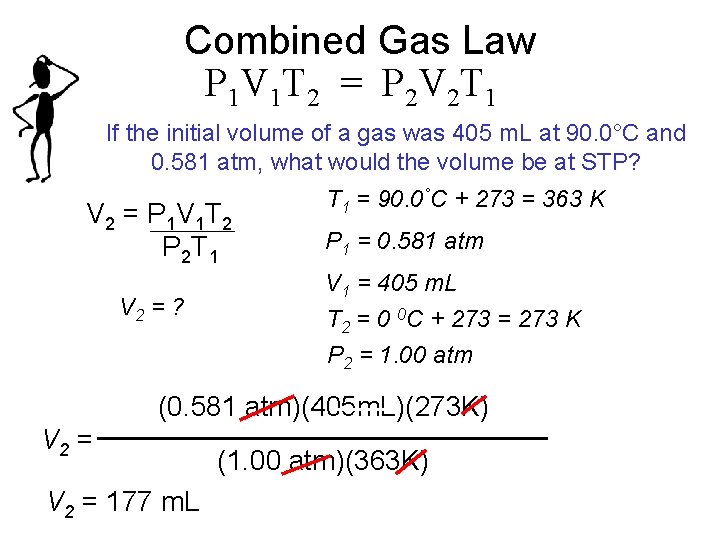
Combined Gas Law P 1 V 1 T 2 = P 2 V 2 T 1 If the initial volume of a gas was 405 m. L at 90. 0°C and 0. 581 atm, what would the volume be at STP? V 2 = P 1 V 1 T 2 P 2 T 1 V 2 = ? T 1 = 90. 0°C + 273 = 363 K P 1 = 0. 581 atm V 1 = 405 m. L T 2 = 0 0 C + 273 = 273 K P 2 = 1. 00 atm (0. 581 atm)(405 m. L)(273 K) V 2 = 177 m. L (1. 00 atm)(363 K)

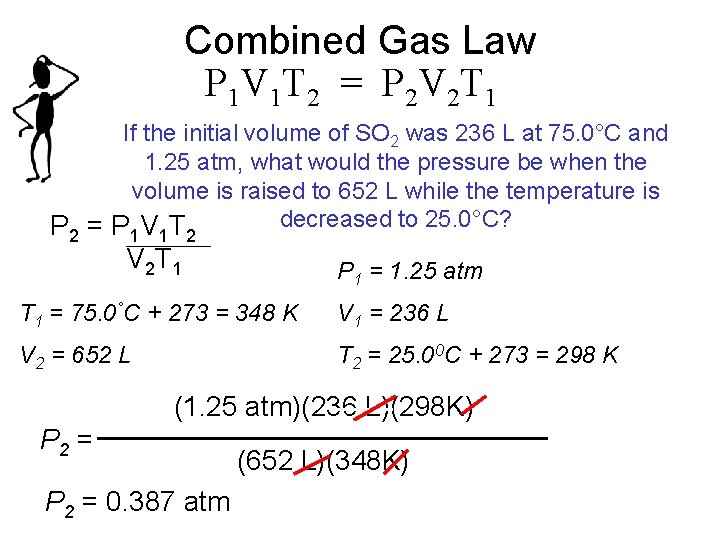
Combined Gas Law P 1 V 1 T 2 = P 2 V 2 T 1 If the initial volume of SO 2 was 236 L at 75. 0°C and 1. 25 atm, what would the pressure be when the volume is raised to 652 L while the temperature is decreased to 25. 0°C? P 2 = P 1 V 1 T 2 V 2 T 1 P 1 = 1. 25 atm T 1 = 75. 0°C + 273 = 348 K V 1 = 236 L V 2 = 652 L T 2 = 25. 00 C + 273 = 298 K (1. 25 atm)(236 L)(298 K) P 2 = 0. 387 atm (652 L)(348 K)

Ideal Gas Law • ideal gas - follows all assumptions of kinetic theory (particles have no volume, no attraction between particles) • used to find one factor that effects gas when the other 3 factors are known – about the gas at one moment in time (no changing conditions) • only law that allows you to determine the amount of gas • PV = n. RT – R is ideal gas law constant

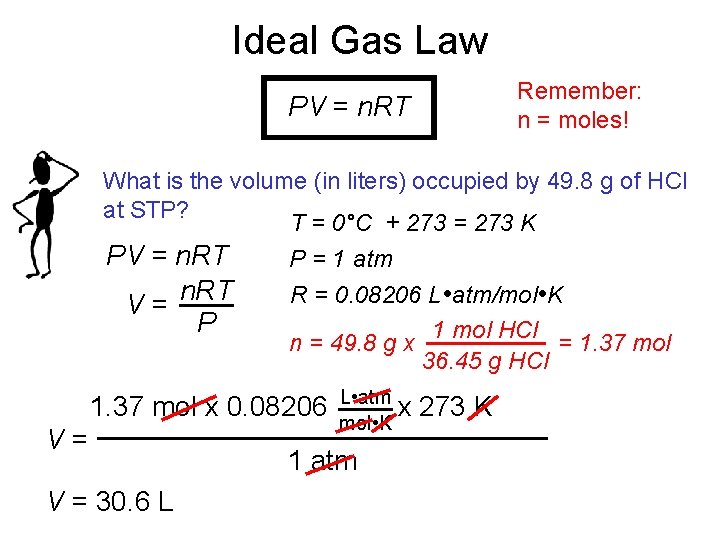
Ideal Gas Law Remember: n = moles! PV = n. RT What is the volume (in liters) occupied by 49. 8 g of HCl at STP? T = 0°C + 273 = 273 K PV = n. RT P = 1 atm n. RT R = 0. 08206 L • atm/mol • K V= P 1 mol HCl n = 49. 8 g x = 1. 37 mol 36. 45 g HCl 1. 37 mol x 0. 08206 V= V = 30. 6 L L • atm x mol • K 1 atm 273 K

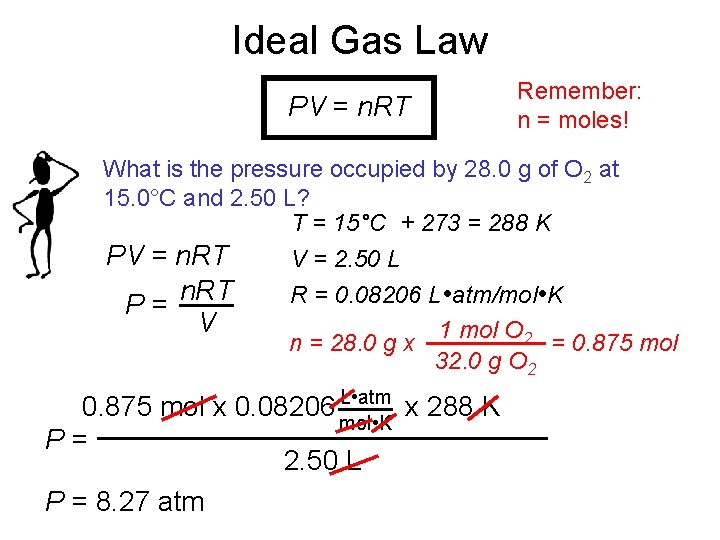
Ideal Gas Law PV = n. RT Remember: n = moles! What is the pressure occupied by 28. 0 g of O 2 at 15. 0°C and 2. 50 L? T = 15°C + 273 = 288 K PV = n. RT V = 2. 50 L n. RT R = 0. 08206 L • atm/mol • K P= V 1 mol O 2 n = 28. 0 g x = 0. 875 mol 32. 0 g O 2 L • atm 0. 875 mol x 0. 08206 mol • K x 288 K P= 2. 50 L P = 8. 27 atm

















