Charless Law CHarless http blog naver combravetop So

Charles's Law CHarles's http: //blog. naver. com/bravetop
So http: //blog. naver. com/bravetop

So http: //blog. naver. com/bravetop

http: //blog. naver. com/bravetop

http: //blog. naver. com/bravetop
http: //blog. naver. com/bravetop

Charles's Law http: //blog. naver. com/bravetop
Charles's Law http: //blog. naver. com/bravetop

http: //blog. naver. com/bravetop

IS http: //blog. naver. com/bravetop
IS http: //blog. naver. com/bravetop
IS http: //blog. naver. com/bravetop
http: //blog. naver. com/bravetop



Charles's Law 1 INTRODUCTION 2 Charles's Law 3 Practice http: //blog. naver. com/bravetop
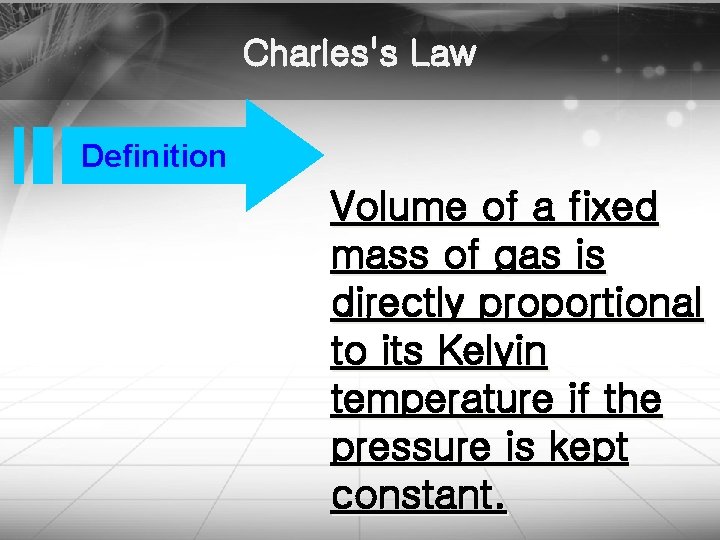
Charles's Law Definition Volume of a fixed mass of gas is directly proportional to its Kelvin temperature if the pressure is kept constant. http: //blog. naver. com/bravetop
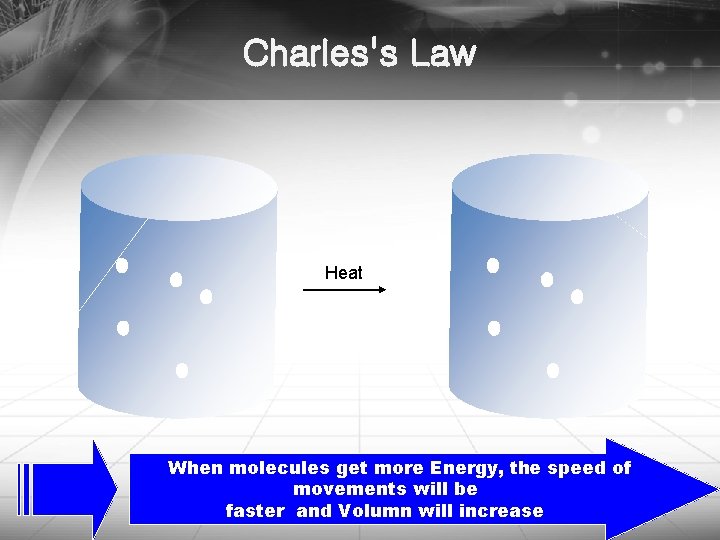
Charles's Law Heat When molecules get more Energy, the speed of movements will be faster and Volumn will increase http: //blog. naver. com/bravetop

Volumn and Temperature High Volumn Low Temperature High http: //blog. naver. com/bravetop

Foumula 1 V 1= Original Volumn T 1=Original Temperature 2 V 2=New Volumn T 2=New Temperature 3 http: //blog. naver. com/bravetop

Practice Question If a sample of gas occupies 6. 80 L at 325 C, what will its volumn be at 25 C if the pressure does not change? 1 V 1= 6. 8 L T 1=325+273= 598 K 2 V 2=? T 2=25+273= 298 K 3 http: //blog. naver. com/bravetop
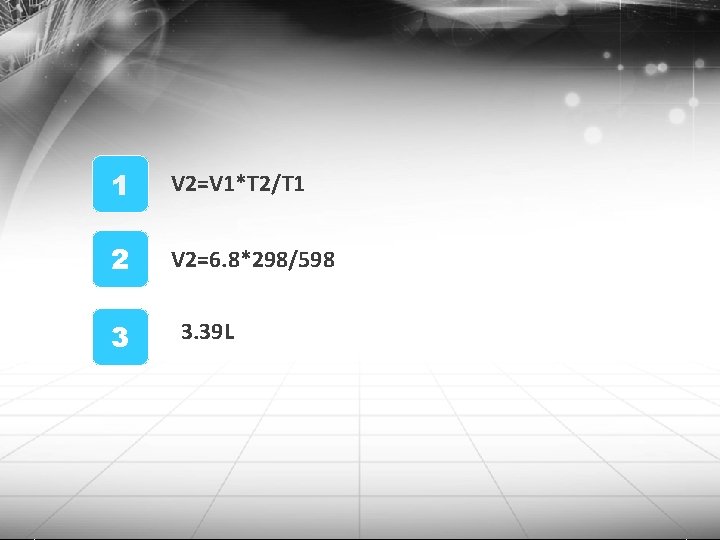
1 V 2=V 1*T 2/T 1 2 V 2=6. 8*298/598 3 3. 39 L http: //blog. naver. com/bravetop

THA NKYOU UOYKNAHT http: //blog. naver. com/bravetop
- Slides: 23