From Epidemic to Elimination Serogroup A Conjugate Meningococcal













- Slides: 35

From Epidemic to Elimination: Serogroup A Conjugate Meningococcal Vaccine for the Meningitis Belt Nancy Messonnier, M. D. Chief, Meningitis and Vaccine Preventable Diseases Branch Division of Bacterial Diseases National Center for Immunization and Respiratory Diseases


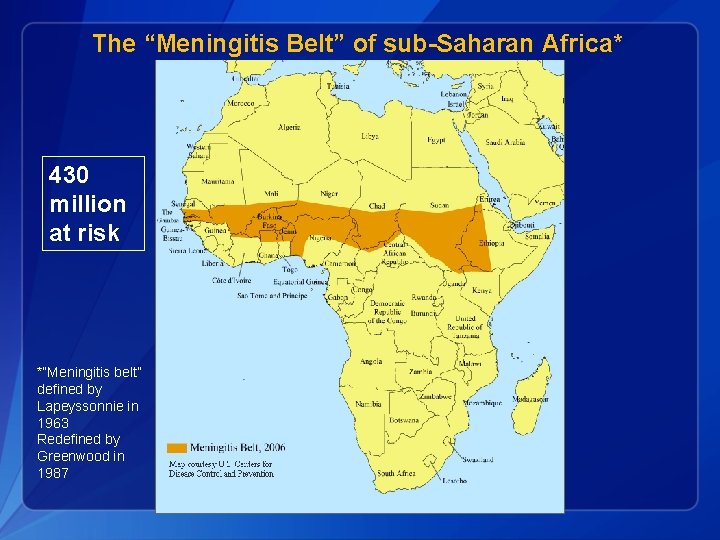
The “Meningitis Belt” of sub-Saharan Africa* 430 million at risk *“Meningitis belt” defined by Lapeyssonnie in 1963 Redefined by Greenwood in 1987

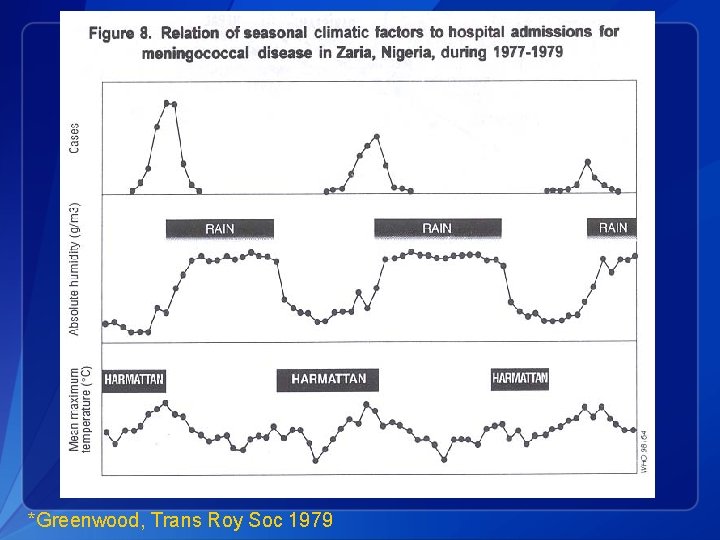
*Greenwood, Trans Roy Soc 1979

Meningococcal Disease Epidemics, Africa - 1970 - 1996* Country Nigeria Rwanda Burkina Faso (Nm. C) Cote d’Ivoire Chad Sudan Kenya Burundi Burkina Faso Nigeria Ghana Year 1977 1978 1979 1983 1988 1989 1992 1996 1997 Cases 1, 257 248 539 414 4, 542 32, 016 3, 800 1, 615 7, 221 10, 089 14, 277 Incidence (per 100, 000) 360 729 517 107 826 133 250 608 1, 249 426 1, 260 *Adapted from Control of Epidemic Meningococcal Disease, WHO Practical Guidelines, 2 nd edition

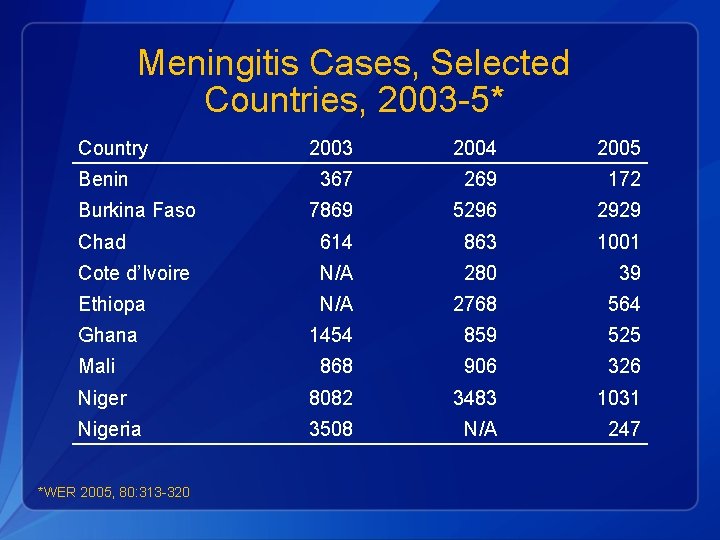
Meningitis Cases, Selected Countries, 2003 -5* Country 2003 2004 2005 367 269 172 7869 5296 2929 Chad 614 863 1001 Cote d’Ivoire N/A 280 39 Ethiopa N/A 2768 564 Ghana 1454 859 525 Mali 868 906 326 Niger 8082 3483 1031 Nigeria 3508 N/A 247 Benin Burkina Faso *WER 2005, 80: 313 -320

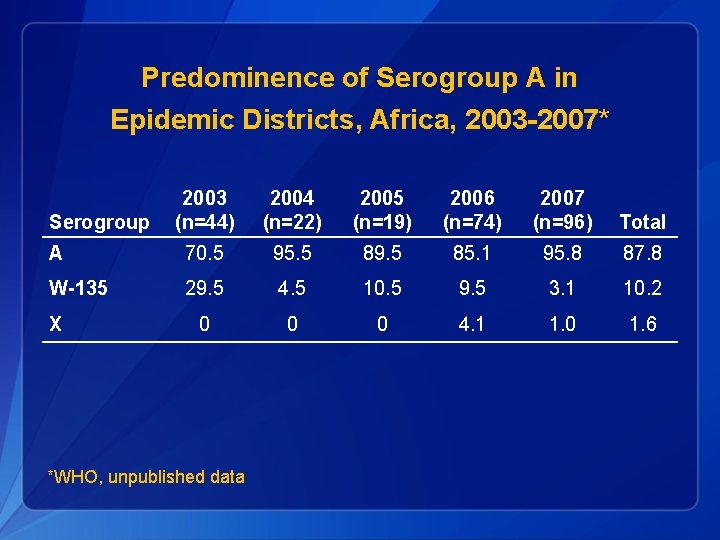
Predominence of Serogroup A in Epidemic Districts, Africa, 2003 -2007* 2003 (n=44) 2004 (n=22) 2005 (n=19) 2006 (n=74) 2007 (n=96) Total A 70. 5 95. 5 89. 5 85. 1 95. 8 87. 8 W-135 29. 5 4. 5 10. 5 9. 5 3. 1 10. 2 0 0 0 4. 1 1. 0 1. 6 Serogroup X *WHO, unpublished data

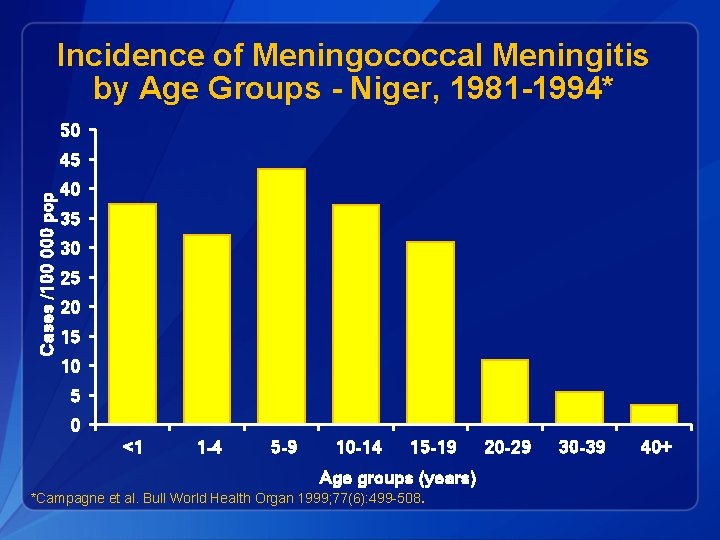
Incidence of Meningococcal Meningitis by Age Groups - Niger, 1981 -1994* 50 Cases /100 000 pop 45 40 35 30 25 20 15 10 5 0 <1 1 -4 5 -9 10 -14 15 -19 Age groups (years) *Campagne et al. Bull World Health Organ 1999; 77(6): 499 -508. 20 -29 30 -39 40+

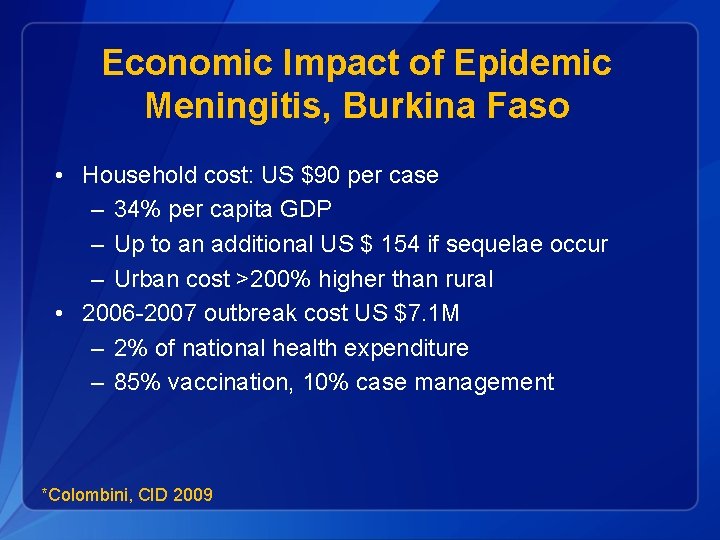
Economic Impact of Epidemic Meningitis, Burkina Faso • Household cost: US $90 per case – 34% per capita GDP – Up to an additional US $ 154 if sequelae occur – Urban cost >200% higher than rural • 2006 -2007 outbreak cost US $7. 1 M – 2% of national health expenditure – 85% vaccination, 10% case management *Colombini, CID 2009

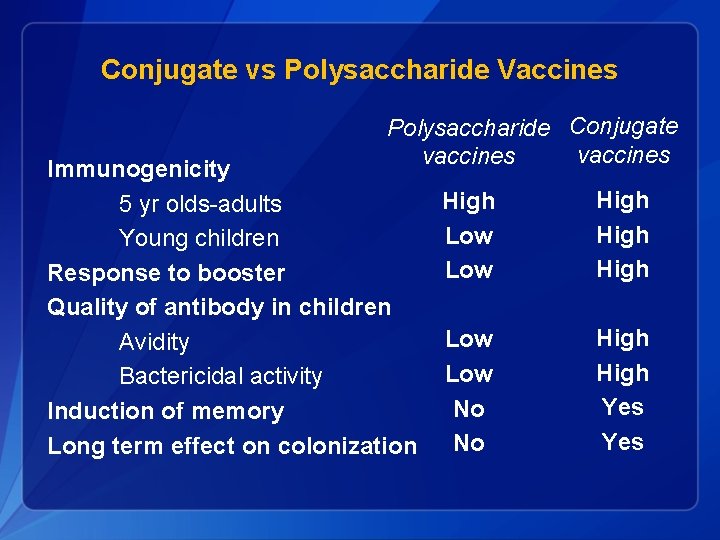
Conjugate vs Polysaccharide Vaccines Polysaccharide Conjugate vaccines Immunogenicity 5 yr olds-adults Young children Response to booster Quality of antibody in children Avidity Bactericidal activity Induction of memory Long term effect on colonization High Low High Low No No High Yes

Meningococcal Conjugate Vaccines for Africa, View from cy 2000 • A/C conjugate vaccine field trials – Gambia (1992 -1995) – Niger (1996 -1997) • Studies on A containing conjugate in Europe/US • Progress and timelines slow • Availability of new funds

The Meningitis Vaccine Project: Novel Approach to Vaccine Development • Partnership between WHO and PATH, with a Grant from Bill and Melinda Gates foundation (2001) • Manufacturer consortium – Syn. Co Bio. Partners (Amsterdam) - Nm. A PS – Statens Serum Institute (Denmark) - TT – FDA-developed conjugation method – Serum Institute of India – vaccine • Price = $0. 40 per dose

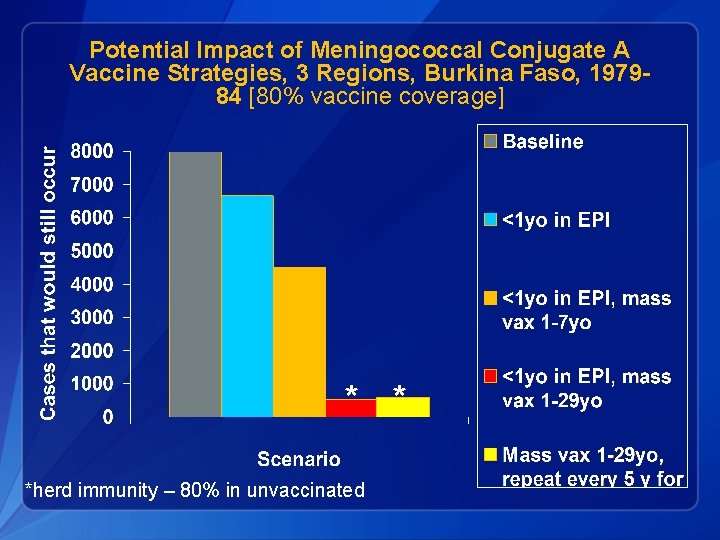
Potential Impact of Meningococcal Conjugate A Vaccine Strategies, 3 Regions, Burkina Faso, 197984 [80% vaccine coverage] * * *herd immunity – 80% in unvaccinated

Men. Afri. Vac – Recent Progress • Good safety profile – About 7000 adults and children vaccinated in India and Africa • Licenced Indian NRA – December 2009 • WHO prequalified – June 2010

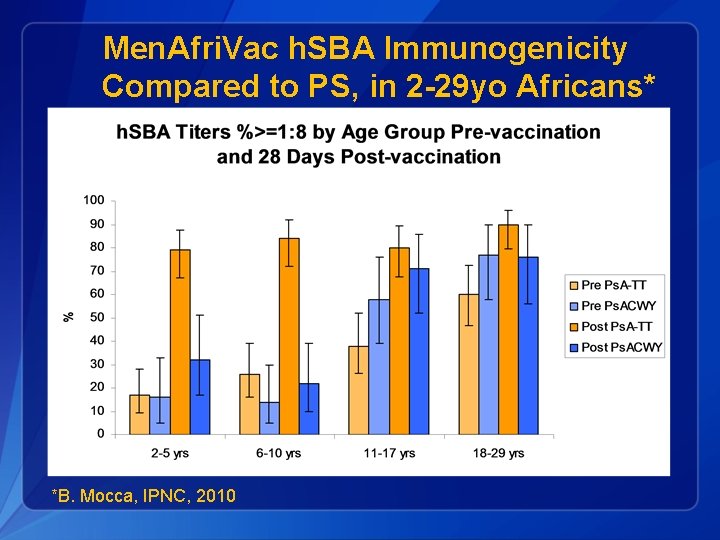
Men. Afri. Vac h. SBA Immunogenicity Compared to PS, in 2 -29 yo Africans* *B. Mocca, IPNC, 2010

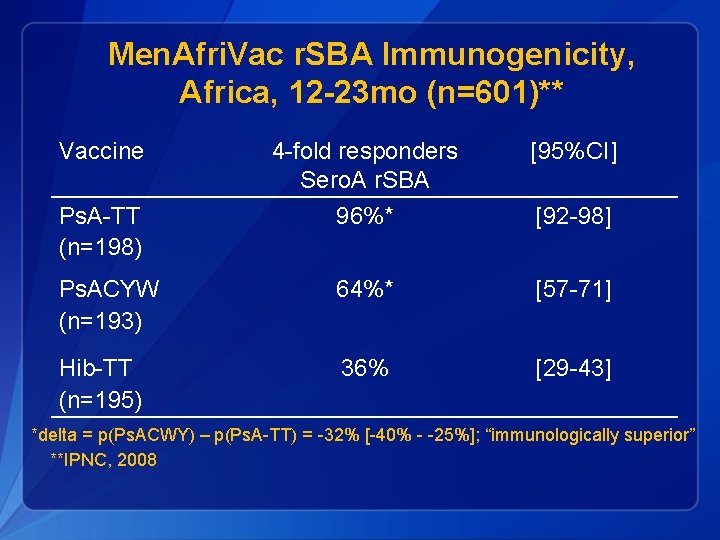
Men. Afri. Vac r. SBA Immunogenicity, Africa, 12 -23 mo (n=601)** Vaccine 4 -fold responders Sero. A r. SBA [95%CI] Ps. A-TT (n=198) 96%* [92 -98] Ps. ACYW (n=193) 64%* [57 -71] Hib-TT (n=195) 36% [29 -43] *delta = p(Ps. ACWY) – p(Ps. A-TT) = -32% [-40% - -25%]; “immunologically superior” **IPNC, 2008

Introduction – Burkina Faso, Mali, Niger • Phase 1: September 2010: – Introduction in a limited population: 400000 doses in each country – Active Surveillance of AEFIs – Assess logistical constraints • Phase 2: December 2010: – Burkina: 11 millions persons – Mali: 4 millions persons – Niger: 4 millions persons • Phase 3: 4 th quarter 2011: – Mali: 7 millions persons – Niger: 8 million persons

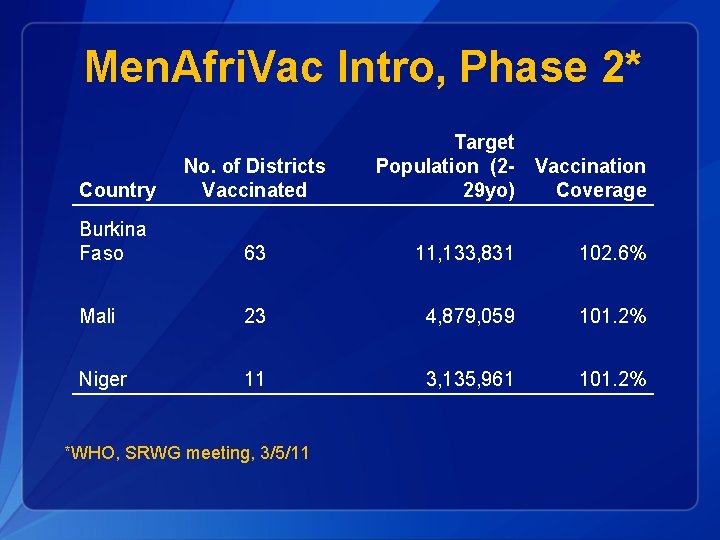
Men. Afri. Vac Intro, Phase 2* Target Population (2 - Vaccination 29 yo) Coverage Country No. of Districts Vaccinated Burkina Faso 63 11, 133, 831 102. 6% Mali 23 4, 879, 059 101. 2% Niger 11 3, 135, 961 101. 2% *WHO, SRWG meeting, 3/5/11

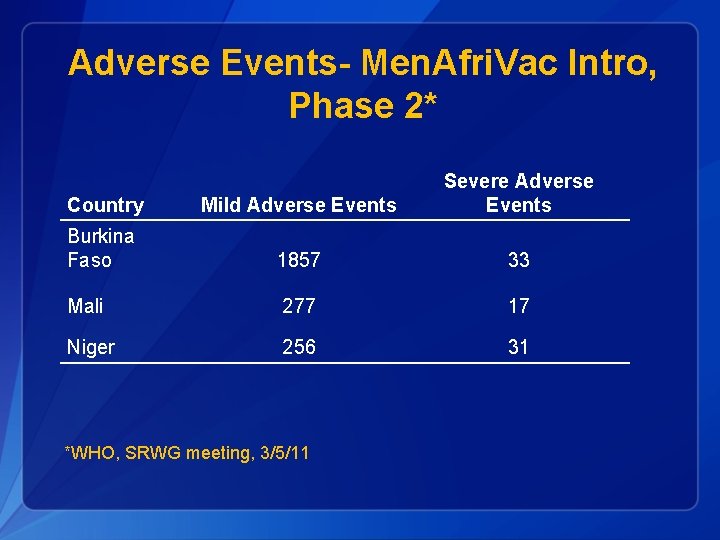
Adverse Events- Men. Afri. Vac Intro, Phase 2* Country Mild Adverse Events Severe Adverse Events Burkina Faso 1857 33 Mali 277 17 Niger 256 31 *WHO, SRWG meeting, 3/5/11

“Early Returns” Men. Afri. Vac Impact 20/28 20/35

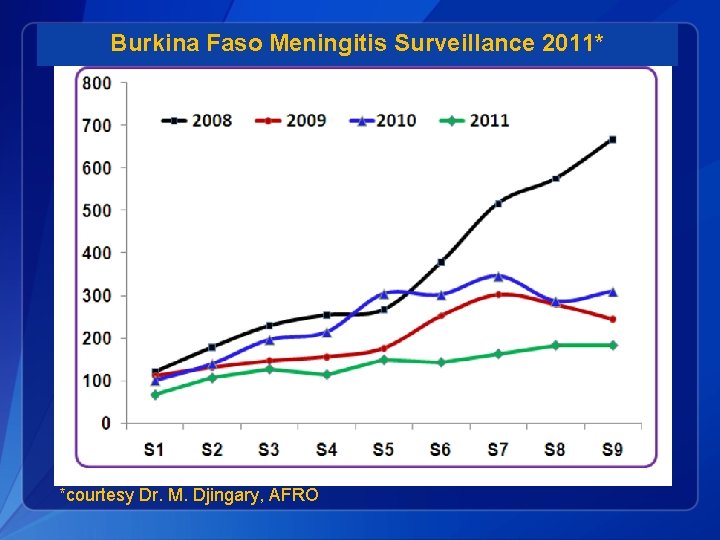
Burkina Faso Meningitis Surveillance 2011* *courtesy Dr. M. Djingary, AFRO

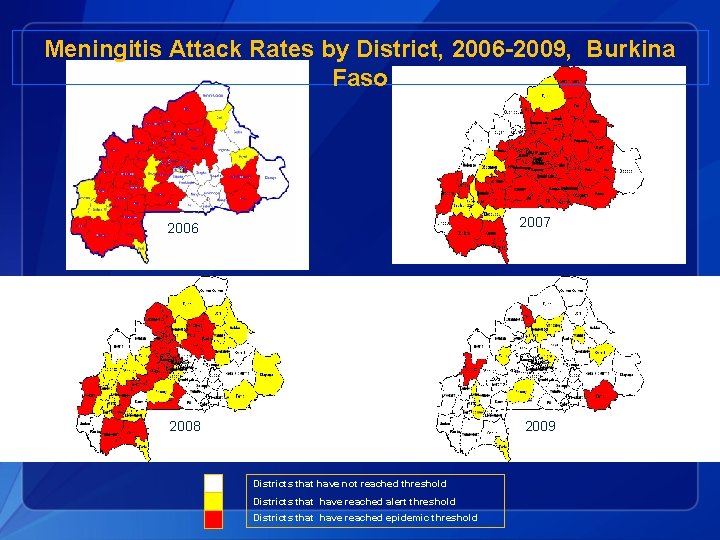
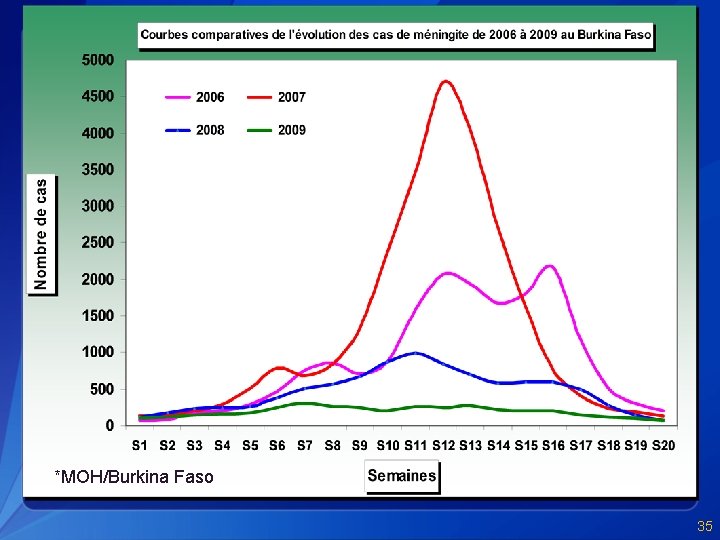
Meningitis Attack Rates by District, 2006 -2009, Burkina Faso 2006 2007 2006 2008 2009 Districts that have not reached threshold Districts that have reached alert threshold Districts that have reached epidemic threshold

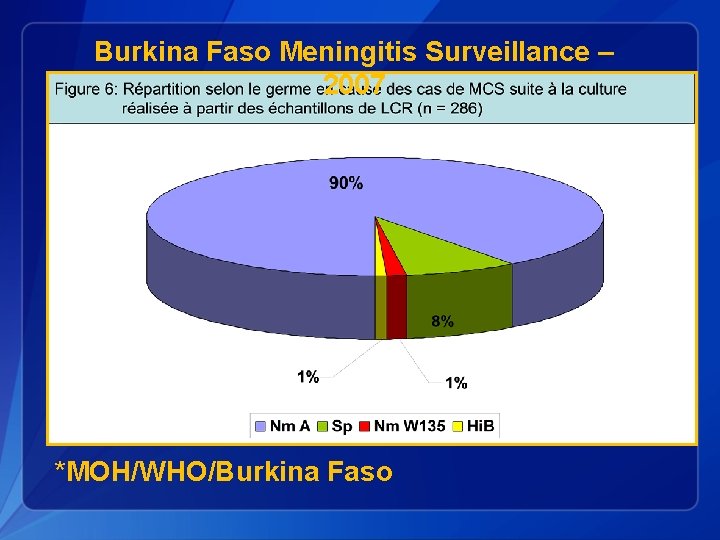
Burkina Faso Meningitis Surveillance – 2007 *MOH/WHO/Burkina Faso

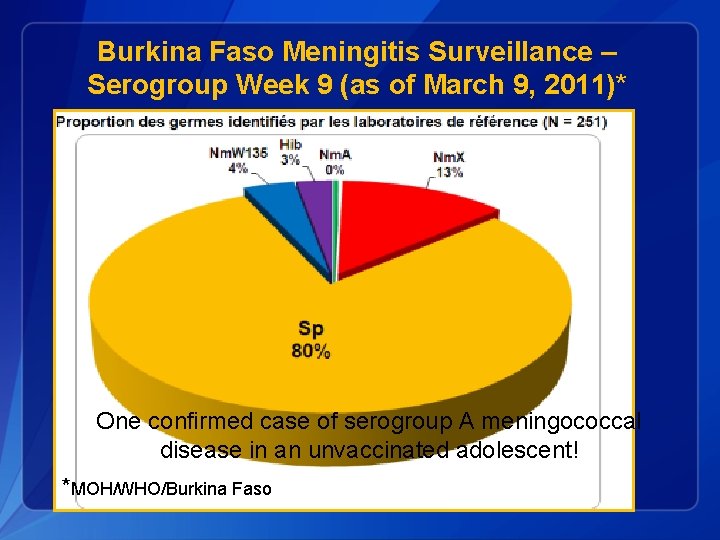
Burkina Faso Meningitis Surveillance – Serogroup Week 9 (as of March 9, 2011)* One confirmed case of serogroup A meningococcal disease in an unvaccinated adolescent! *MOH/WHO/Burkina Faso

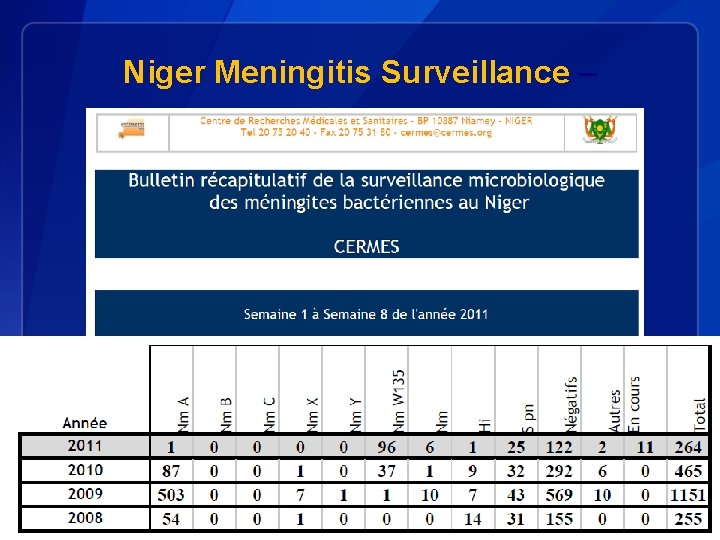
Niger Meningitis Surveillance –

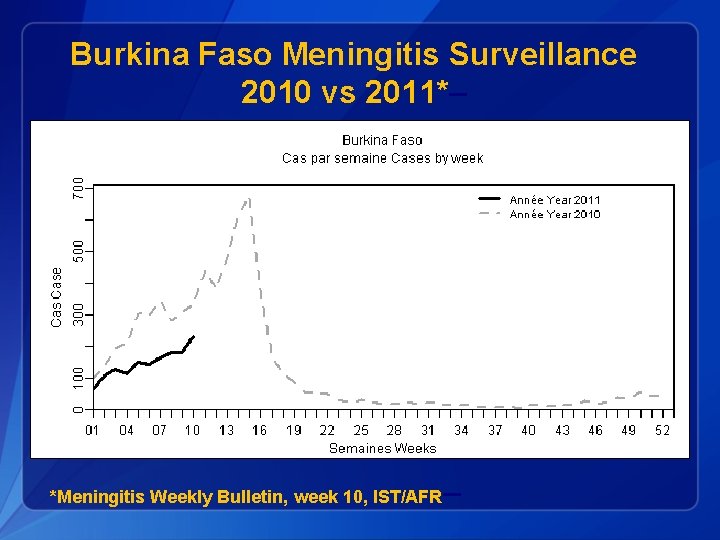
Burkina Faso Meningitis Surveillance 2010 vs 2011*– *Meningitis Weekly Bulletin, week 10, IST/AFR –


Post Licensure Impact Assessment • • Impact on outbreaks Field effectiveness Vaccine safety Correlates of protection Indirect effects of vaccination Duration of protection Impact on molecular epidemiology Impact of variations in programs

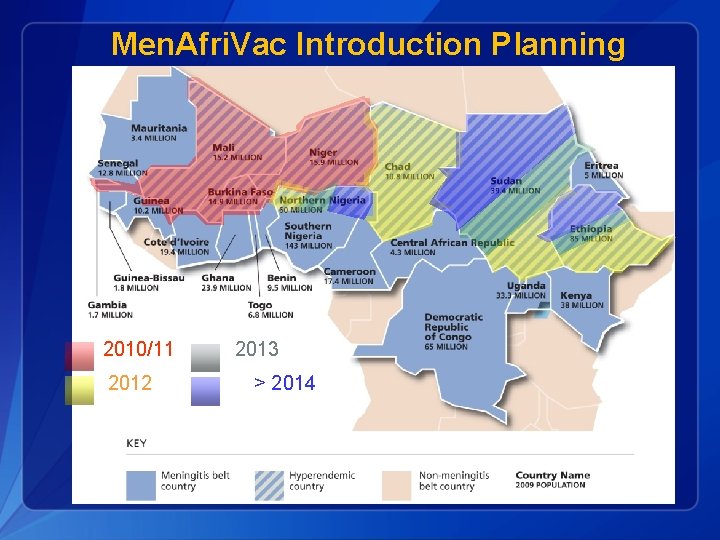
Men. Afri. Vac Introduction Planning 2010/11 2012 2013 > 2014

*courtesy M. La. Force

Elimination of Epidemic Meningitis is Possible, with Good Partners!

Extra slides

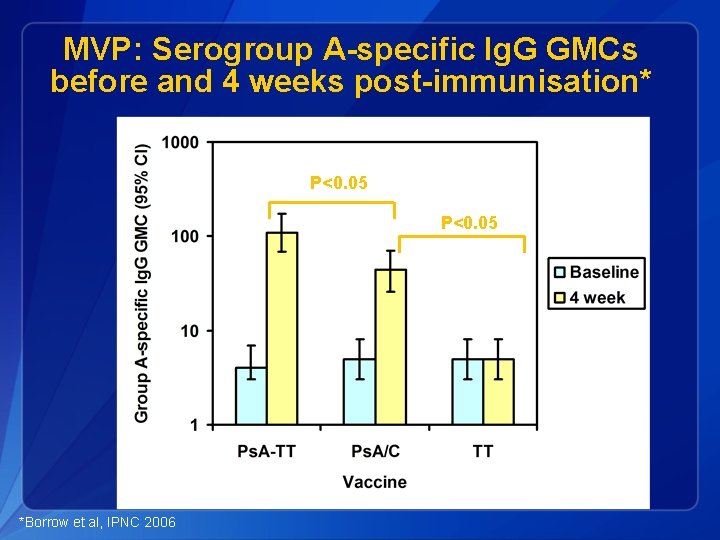
MVP: Serogroup A-specific Ig. G GMCs before and 4 weeks post-immunisation* P<0. 05 *Borrow et al, IPNC 2006

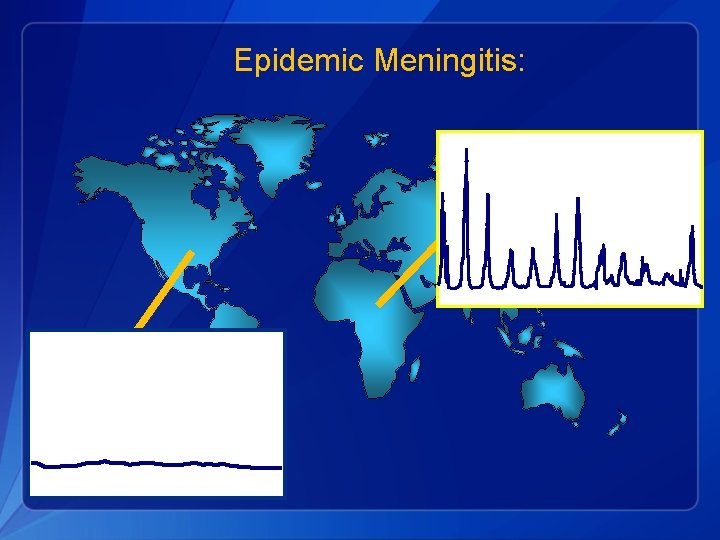
Epidemic Meningitis: A, C B, C

*MOH/Burkina Faso 35
 L. pneumophila serogroup 1 in a water sample
L. pneumophila serogroup 1 in a water sample Invasive meningococcal disease
Invasive meningococcal disease Endemic epidemic
Endemic epidemic Epidemic dropsy
Epidemic dropsy Aids epidemic
Aids epidemic Epidemic dropsy
Epidemic dropsy Endemic epidemic
Endemic epidemic Doctors attributed the epidemic to the rampant
Doctors attributed the epidemic to the rampant Epidemiological transition model
Epidemiological transition model Epidemic broadcast trees
Epidemic broadcast trees Row operation method
Row operation method Conjugate voyager
Conjugate voyager Length of transverse axis of hyperbola
Length of transverse axis of hyperbola Simplify a surd
Simplify a surd Conjugate amo
Conjugate amo Ayudar conjugation
Ayudar conjugation Conjugate croire
Conjugate croire Linear factors theorem and conjugate zeros theorem
Linear factors theorem and conjugate zeros theorem A double point is called cusp of tangents at this point are
A double point is called cusp of tangents at this point are Conjugate principal point in aerial photography
Conjugate principal point in aerial photography Spanish verb
Spanish verb Amphoteric substances
Amphoteric substances Conjugate llegar
Conjugate llegar Four conic sections
Four conic sections Jugar conjugation
Jugar conjugation Correction
Correction Conjugate heat transfer ansys
Conjugate heat transfer ansys Conjugate conocer
Conjugate conocer Conjuguemos
Conjuguemos Conjugate acid base pair example
Conjugate acid base pair example Is levantarse a reflexive verb
Is levantarse a reflexive verb Hocl conjugate base
Hocl conjugate base Conjugate pair of 2√5-5√2
Conjugate pair of 2√5-5√2 Ar infinitives
Ar infinitives Conjugate base of methylamine
Conjugate base of methylamine Futur proche aller negative
Futur proche aller negative