Formula Mass of Compounds Molar mass of an




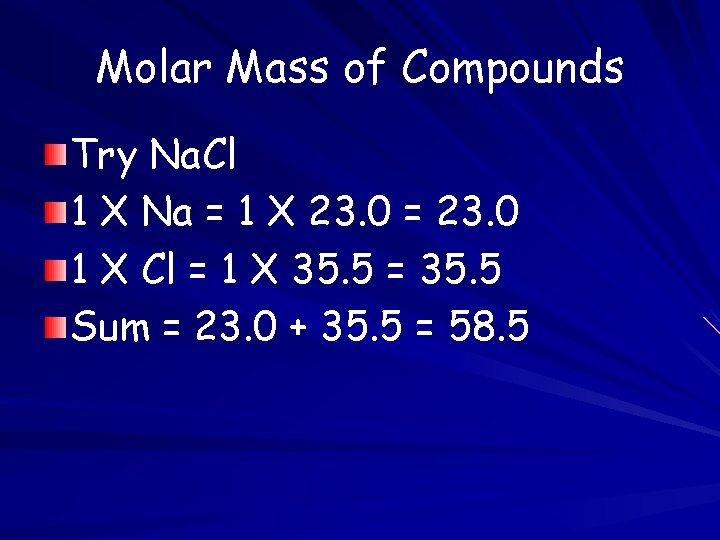
- Slides: 11

Formula Mass of Compounds

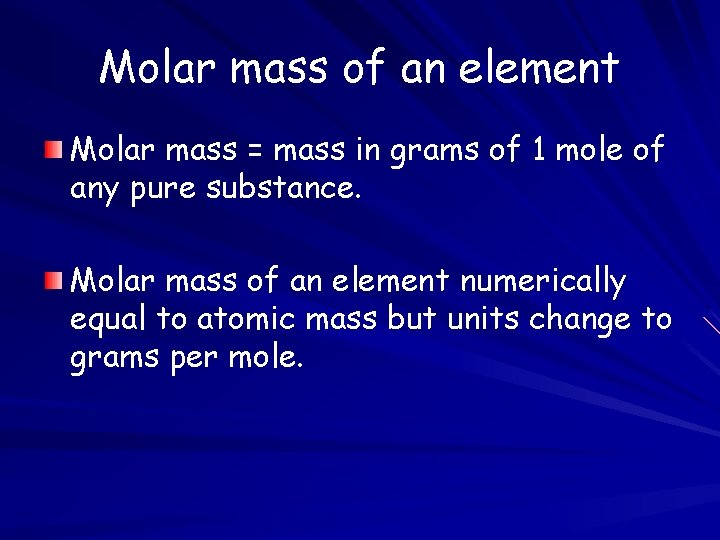
Molar mass of an element Molar mass = mass in grams of 1 mole of any pure substance. Molar mass of an element numerically equal to atomic mass but units change to grams per mole.

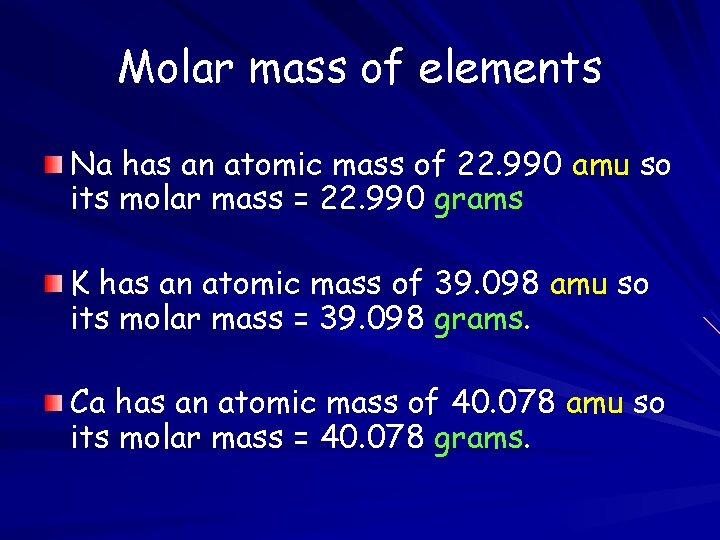
Molar mass of elements Na has an atomic mass of 22. 990 amu so its molar mass = 22. 990 grams K has an atomic mass of 39. 098 amu so its molar mass = 39. 098 grams. Ca has an atomic mass of 40. 078 amu so its molar mass = 40. 078 grams.

Molar mass Also called formula mass. Or gram formula mass.

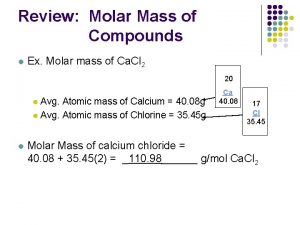
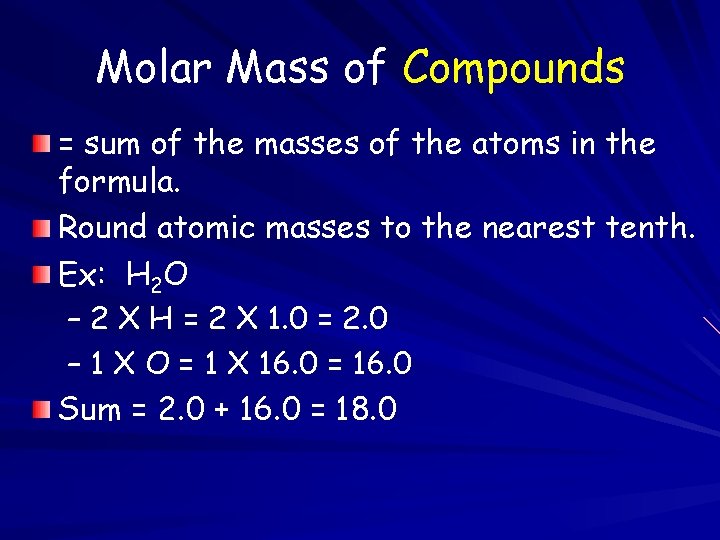
Molar Mass of Compounds = sum of the masses of the atoms in the formula. Round atomic masses to the nearest tenth. Ex: H 2 O – 2 X H = 2 X 1. 0 = 2. 0 – 1 X O = 1 X 16. 0 = 16. 0 Sum = 2. 0 + 16. 0 = 18. 0

Molar Mass of Compounds Try CH 4 1 X C = 1 X 12. 0 = 12. 0 4 X H = 4 X 1. 0 = 4. 0 Sum = 4. 0 + 12. 0 = 16. 0

Molar Mass of Compounds Try CO 2 1 X C = 1 X 12. 0 = 12. 0 2 X O = 2 X 16. 0 = 32. 0 Sum = 12. 0 + 32. 0 = 44. 0
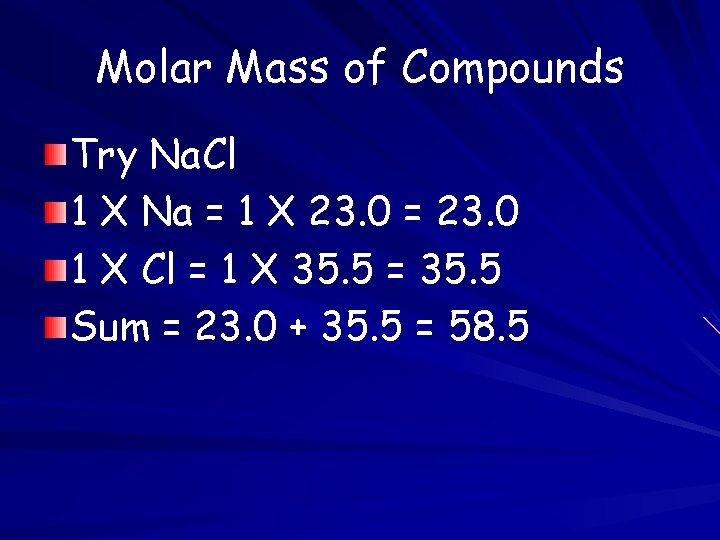
Molar Mass of Compounds Try Na. Cl 1 X Na = 1 X 23. 0 = 23. 0 1 X Cl = 1 X 35. 5 = 35. 5 Sum = 23. 0 + 35. 5 = 58. 5

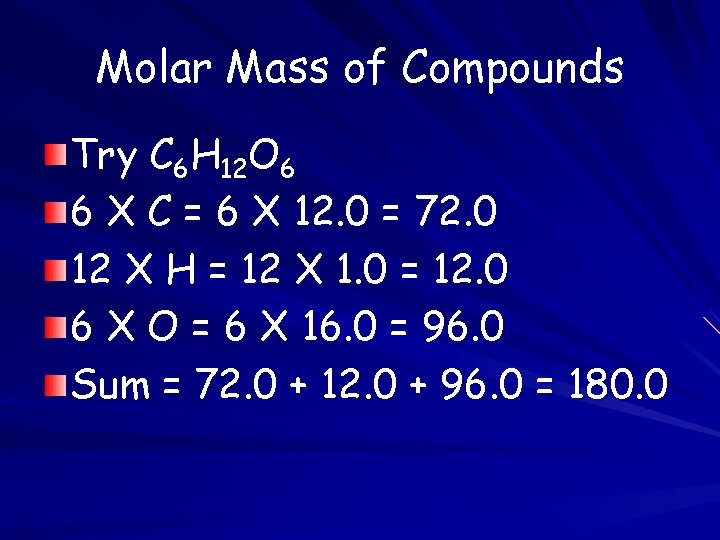
Molar Mass of Compounds Try C 6 H 12 O 6 6 X C = 6 X 12. 0 = 72. 0 12 X H = 12 X 1. 0 = 12. 0 6 X O = 6 X 16. 0 = 96. 0 Sum = 72. 0 + 12. 0 + 96. 0 = 180. 0

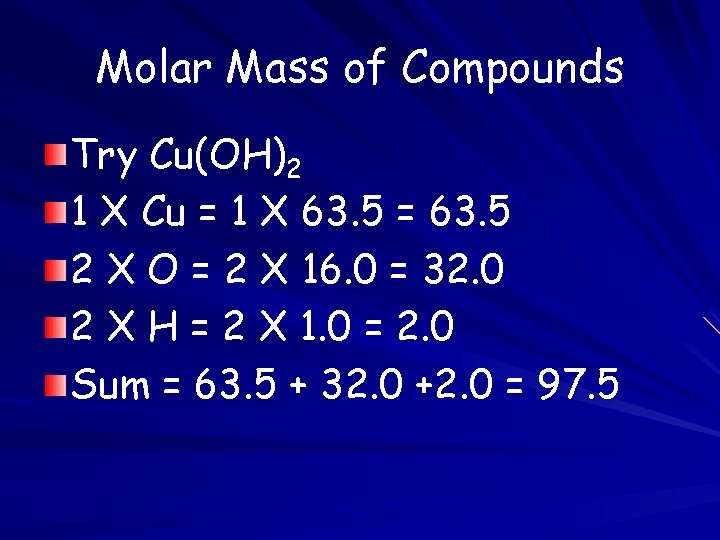
Molar Mass of Compounds Try Cu(OH)2 1 X Cu = 1 X 63. 5 = 63. 5 2 X O = 2 X 16. 0 = 32. 0 2 X H = 2 X 1. 0 = 2. 0 Sum = 63. 5 + 32. 0 +2. 0 = 97. 5

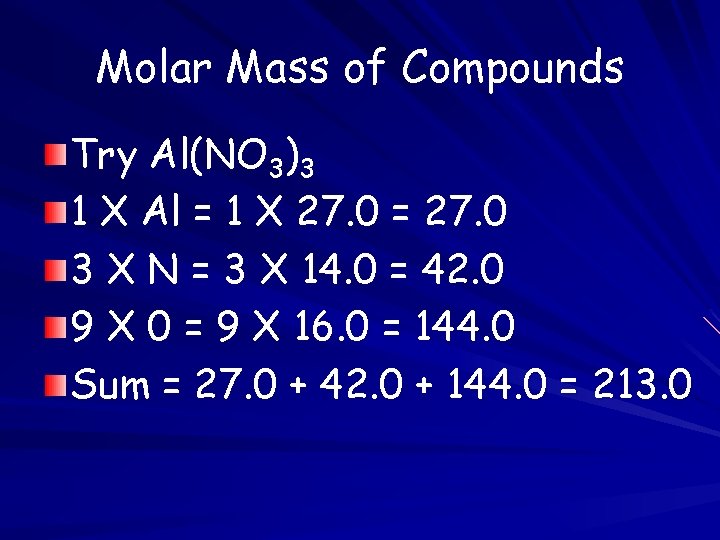
Molar Mass of Compounds Try Al(NO 3)3 1 X Al = 1 X 27. 0 = 27. 0 3 X N = 3 X 14. 0 = 42. 0 9 X 0 = 9 X 16. 0 = 144. 0 Sum = 27. 0 + 42. 0 + 144. 0 = 213. 0