Field Trip 1 Hocking Hills State Park Saturday











- Slides: 19

Field Trip #1: Hocking Hills State Park Saturday, October 2 nd

MINERALS: The Building Blocks of Rocks

Definition of a Mineral • A Mineral is a naturally occurring, inorganic, solid, crystalline substance, with definite physical and chemical properties. • Inorganic – not composed of Carbon – Hydrogen molecules (usually part of living tissue) • Crystalline – atoms have a structured arrangement.

CRYSTAL SHAPES

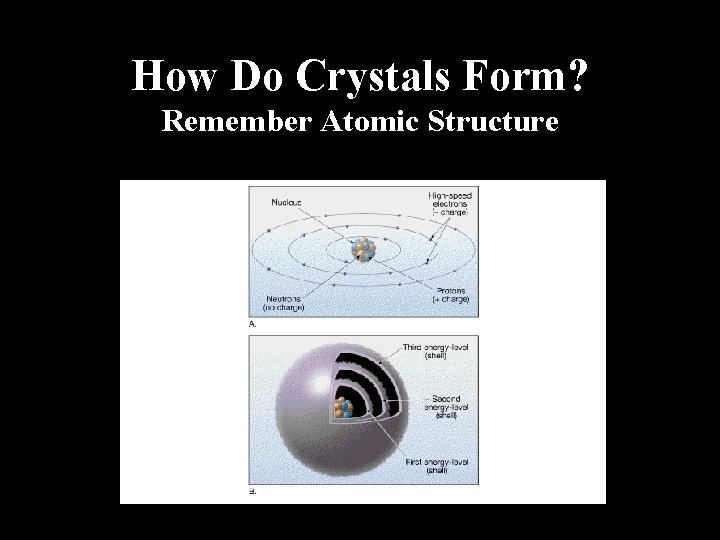
How Do Crystals Form? Remember Atomic Structure

ATOMS DO NOT WANT TO BE NEUTRAL THEY WANT TO BE STABLE § Stable Means Having Their Outer Shell Of Electrons Full To Capacity § This Can Happen By Losing Or Gaining Electrons to Other Atoms §This Process causes Atoms to Join Together: A Process Called Bonding

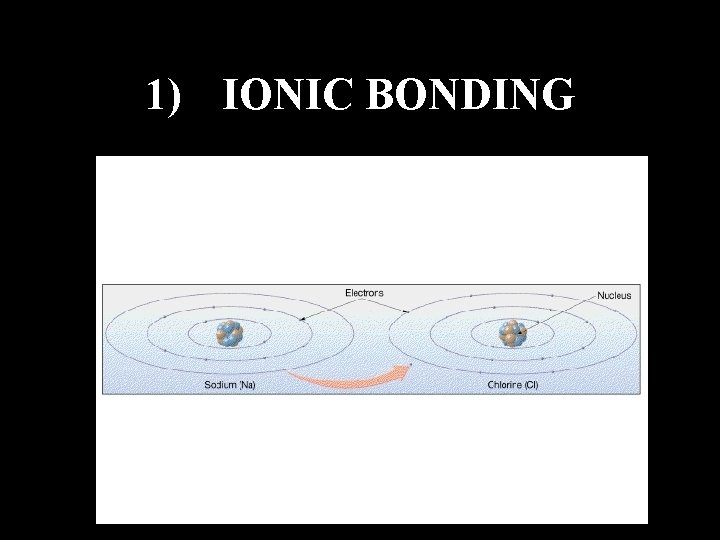
1) IONIC BONDING

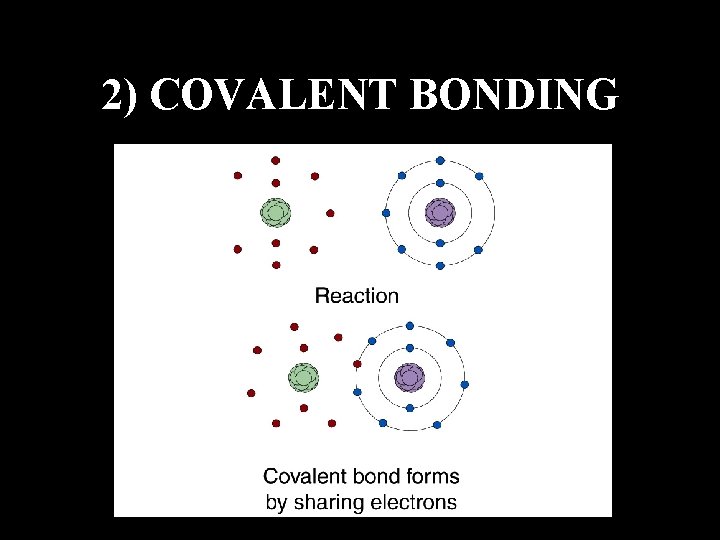
2) COVALENT BONDING

OTHER BONDS 3) Metallic – Electrons act as a mobile cloud that moves from atom to atom. Accounts for excellent electrical conductivity of metals Example: Copper 4) van der Waals – Very weak Bond due to slight polarity of charge on atoms

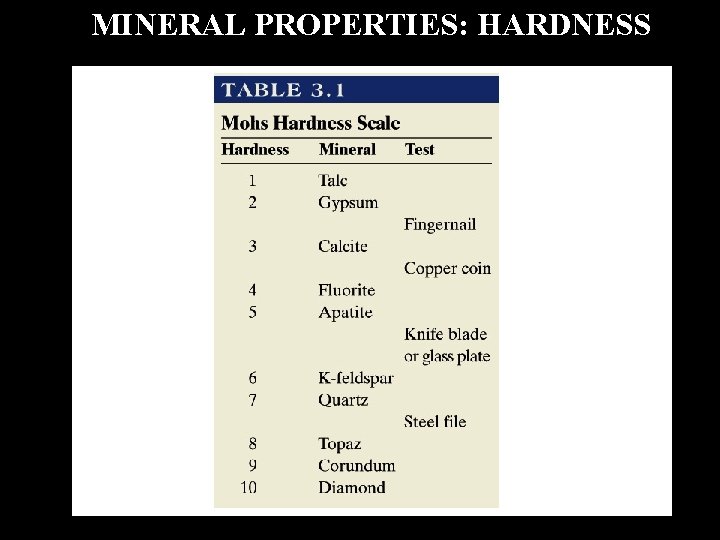
MINERAL PROPERTIES: HARDNESS

MINERAL PROPERTIES: CLEAVAGE Breakage along planes of crystal weakness

MINERAL PROPERTIES: Other Properties §Color §Streak – color of powdered mineral §Luster – the way light reflects of a mineral §Specific Gravity – Weight of Mineral Weight of same volume of water §Fracture – breakage through crystal §Reactivity – Reacts with Acid?

MAJOR MINERAL GROUPS What are the most common minerals in the Crust? 1) What are the most common elements?


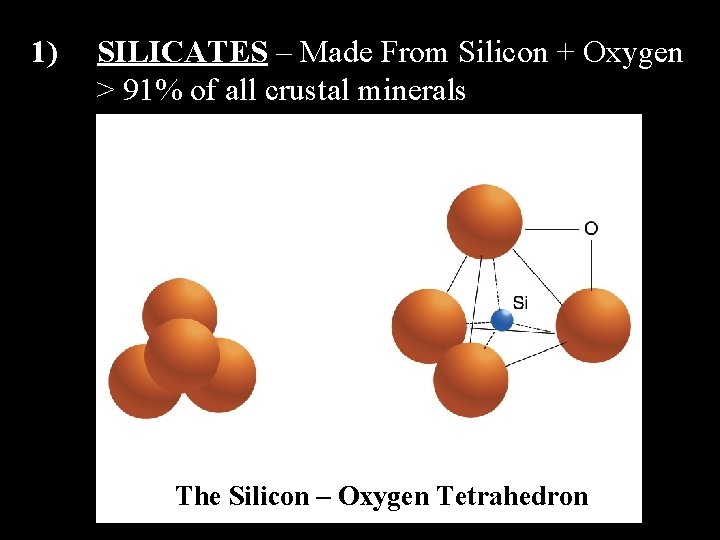
1) SILICATES – Made From Silicon + Oxygen > 91% of all crustal minerals The Silicon – Oxygen Tetrahedron

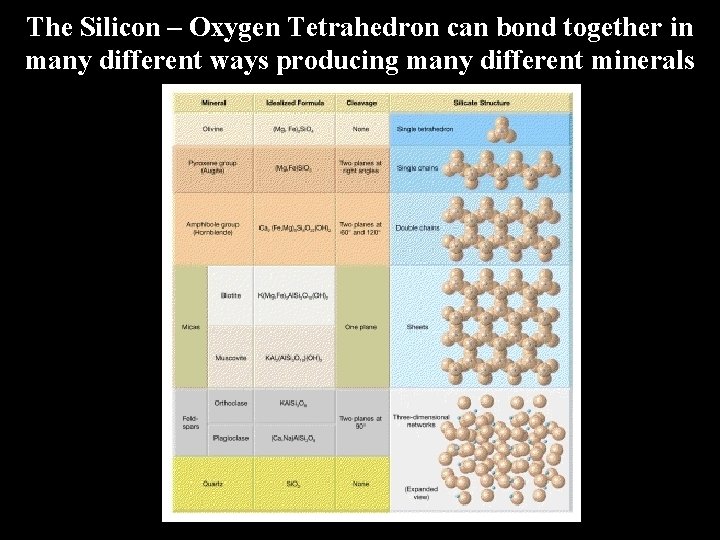
The Silicon – Oxygen Tetrahedron can bond together in many different ways producing many different minerals

Other Important Mineral Groups 2) Carbonates – based on Carbonate ion (CO 3) Calcite - Ca CO 3, Dolomite - Ca. Mg (CO 3)2 Main component of the rocks limestone & dolostone. 3) Oxides – usually metals + oxygen Hematite - Fe 2 O 3 (Iron Oxide or Rust), Corundum - Al 2 O 3 (Aluminum Oxide or Ruby). Excellent source of ore metals

Other Important Mineral Groups 4) Sulfides – based on sulfide ion (S 2 -) Pyrite - Fe. S 2 (“Fool’s Gold”) Excellent source of ore metals 5) Sulfates – based on sulfate ion (SO 4)Gypsum - Ca. SO 4 (Drywall) Used in the construction industry

So, why study minerals? 1) Building Blocks of Rocks – what the Earth is made of. 2) Important Economically – Industry & Agriculture. 3) Can be incredibly beautiful!!
 Katie hocking
Katie hocking Billy once upon a time
Billy once upon a time Travel recount
Travel recount Gilwell park accommodation
Gilwell park accommodation Csudh financial aid office
Csudh financial aid office Csudh mascot
Csudh mascot Virtual field trip to amazon rainforest
Virtual field trip to amazon rainforest Compound word for field
Compound word for field Academic field trip plan a plan b
Academic field trip plan a plan b Field visit definition
Field visit definition Be vape free virtual field trip
Be vape free virtual field trip A trip westward question and answer
A trip westward question and answer Ice picks readworks answer key
Ice picks readworks answer key Virtual field trip salem witch trials
Virtual field trip salem witch trials Shakespeare virtual field trip
Shakespeare virtual field trip What is deposition
What is deposition Intraspm
Intraspm Orchard park community center
Orchard park community center Zachary youth park field map
Zachary youth park field map Plainedge park field
Plainedge park field