FDA Medical Device Review DENNIS R HOCK INVESTIGATOR

































- Slides: 36

FDA Medical Device Review DENNIS R. HOCK, INVESTIGATOR

Topics of Discussion Reorganization of FDA-ORA (Program Realignment) Foreign vs Domestic Inspections, “Is there a difference”? FDA form 483, “The Elephant in the room”.

Program Realignment What is FDA's Program Alignment? Program Alignment is a plan to modernize and strengthen the Food and Drug Administration’s (FDA) workforce to improve public health response in a way that keeps pace with the acceleration of scientific innovation, global expansion of markets, and new programmatic mandates.

Program Realignment Central Region Northeast Region Pacific Region Southeast Region Southwest Region

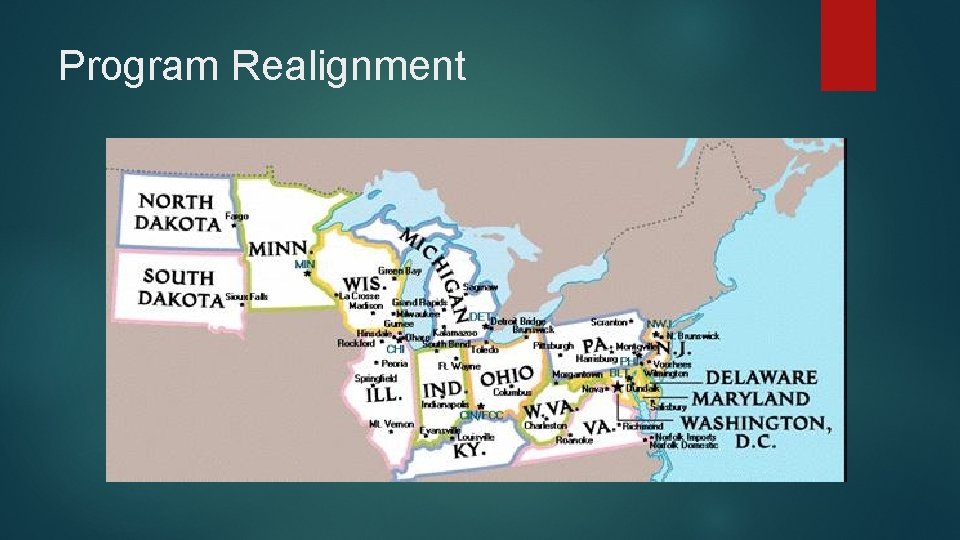
Program Realignment Central Region The Central Region was comprised of Delaware, Illinois, Indiana, Kentucky, Maryland, Michigan, Minnesota, New Jersey, North Dakota, Ohio, Pennsylvania, South Dakota, Virginia, West Virginia, Wisconsin, and the District of Columbia. With seven domestic District Offices located in Baltimore, Chicago, Cincinnati, Detroit, Minneapolis, Newark and Philadelphia.

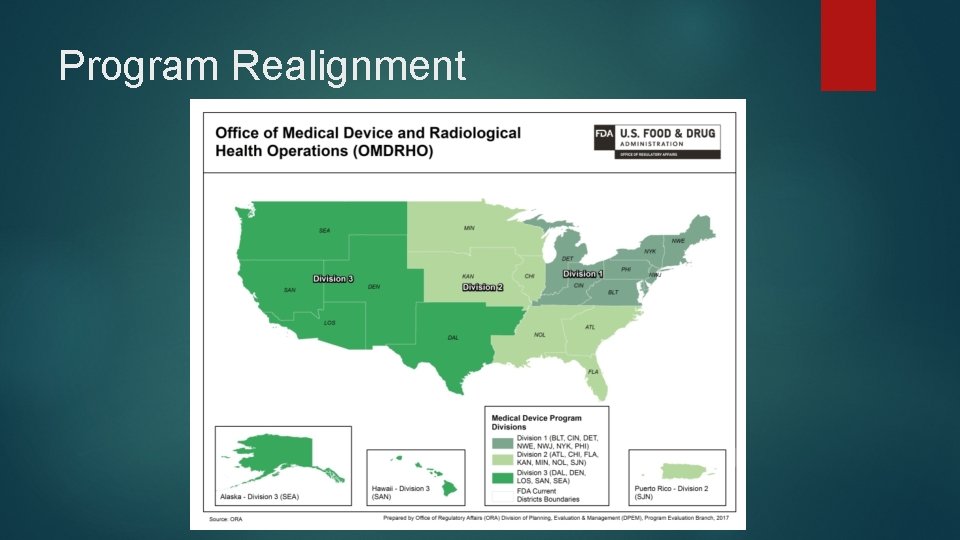
Program Realignment

Program Realignment On May 15, 2017, as part of the broader agency Program Alignment initiative, the U. S. Food and Drug Administration’s (FDA) Office of Regulatory Affairs (ORA) implemented a program-based management structure that aligns staff by FDA-regulated product.

Program Realignment Foods Pharmaceuticals Medical Devices Biologics BIMO’s Twelve (12) Divisions Four (4) Divisions Three (3) Divisions Two (2) Divisions Imports Five (5) Divisions

Program Realignment Divisions Division 1 - Medical Device and Radiological Health East Division 2 - Medical Device and Radiological Health Central Division 3 - Medical Device and Radiological Health West

Program Realignment

Program Realignment Division 1 - Medical Device and Radiological Health East Comprised of six investigation groups Operate as a program specific entity utilizing the same DIB, CB and DD (chain of command) Investigators Division may travel by assignment/workload obligations throughout the

Foreign vs Domestic Inspections, “Is there a difference”?

Foreign vs Domestic Inspections § Foreign Inspections are typically four or four and a half days § Condensed, heavy workload with little time to provide supportive evidence or corrective action against observations § High level review with concentration in specific areas if violations are observed § Regulatory reaction is swift, halting subsequent shipments almost immediately under Import Alerts § Heavily scrutinized typically because of inspectional time lines

Foreign vs Domestic Inspections Example Observations During Foreign Inspection

Foreign vs Domestic Inspections A process whose results cannot be fully verified by subsequent inspection and test has not been adequately validated according to established procedures. 21 CFR 820. 75(a) Specifically, the manual solvent bonding validation, Using Dichlo as bonding agent and to be E-beam Sterilized is not adequate in that it does not provide the individuals that performed the bonding activities during the validation and does not demonstrate that the operators performing the solvent bonding were certified to perform this function. In addition, your procedure used for bonding verification/validation activities, does not provide requirements to include personnel training verification.

Foreign vs Domestic Inspections Required records were not made readily available for review and copying by FDA. 21 CFR 820. 180 Specifically, you did not interpret required, critical records in the appropriate language to make them readily available for review and copying by FDA. As a result, the inspection was not able to be completed.

Foreign vs Domestic Inspections § Inspections can be longer, depending on findings and the scope of the assignment § Workload is less time dependent with more time to provide supportive evidence or corrective action against observations § Depending upon inspectional history, abbreviated inspections are preferred § Regulatory reaction is broad with more time for discussions between the agency and firm management

Foreign vs Domestic Inspections Regulatory Reactions Domestic Inspections Foreign Inspections Untitled Letter Import Alert Regulatory Meeting Detentions at Port Warning Letter Civil Money Penalties Regulatory Meeting Seizure (if in commerce) T. R. O. Injunction

Inspection Observations FDA form 483, “The Elephant in the room”.

Inspection Observations Q: A: What is the purpose of an FDA Form 483? The FDA Form 483 formally documents the company’s management of significant objectionable conditions. Companies are encouraged to respond to the FDA Form 483 in writing with their corrective action plan and then implement that corrective action plan expeditiously.

Inspection Observations Q: A: When is an FDA Form 483 issued? An FDA Form 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed any conditions that in their judgment may constitute violations of the Food Drug and Cosmetic (FD&C) Act and related Acts.

Inspection Observations Q: Is the FDA Form 483 intended to be an allinclusive list of every possible deviation? A: No. There may be other objectionable conditions that exist at the firm that are not cited on the FDA Form 483. FDA investigators are instructed to note only what they saw during the course of the inspection.

Inspection Observations Q: What are the implications of the FDA Form 483 for agency enforcement and what happens next? A: The FDA Form 483 does not constitute a final Agency determination of whether any condition is in violation of the FD&C Act or any of its relevant regulations. The FDA Form 483 is considered, along with a written report, all evidence or documentation collected on-site, and any responses made by the company. The Agency considers all of this information and then determines what further action, if any, is appropriate to protect public health.

Inspection Observations EXERCISE

Inspection Observations Radiofrequency Lesion Generator A Class II medical device, introduced in the 1990’s, typically used in pain clinics or procedures such as Radiofrequency ablation

Inspection Observations CFR says…. . Where servicing is a specified requirement, each manufacturer shall establish and maintain instructions and procedures for performing and verifying that the servicing meets the specified requirements. 820. 200 (a)

Inspection Observations Scenario: The current monthly sales are approximately 10 units with a total of active devices fielded to be about 2000. The firm has a servicing department with an annual rate <10% of the units fielded. 2000 units fielded/~10% servicing rate = approximately 200 units annually serviced or about 16 -18 per month. The firm does not have procedures in place performing and verifying that the servicing meets the specified requirements.

Inspection Observations Between 03/24/2014 and 12/08/2014 (~8 months), the firm serviced 54 units for Printed Circuit Board (PCB) replacement. So, 54 units for PCB replacement, out of ~128 units annually, is about 42%

Inspection Observations Out of the 54 units serviced for PCB replacement, 9 units were manufactured between 09/2013 and 11/2013. Approximately 120 units are manufactured annually, so the 9 units manufactured between 09/2013 and 11/2013 was actually 30%.

Inspection Observations Is this Significant?

Inspection Observations Would you issue a 483?

Inspection Observations Procedures or instructions for performing servicing activities and verifying that servicing meets specified requirements have not been established. 820. 200(a) Specifically, you frequently service for repair your ____ Radiofrequency Lesion Generator and components. Beginning on 03/24/2014 through 12/08/2014, you serviced for repair serial #’s 1 -X, of the _____ Radiofrequency Lesion Generator. You do not have specified requirements established through instruction or procedures, to conduct servicing or testing on the device, to ensure proper function.

Inspection Observations So what Happened?

Inspection Observations Firm established IPC-A-610 as the standard for their incoming acceptance of components. The PCB in question is classified as a Class III critical component. The firm conducted a skip lot acceptance. During the manufacturing period 09/2013 and 11/2013, the firm accepted PCB’s with edge delamination, which should have been 100% rejected.

Inspection Observations The firm segregated the complaints for PCB failures into separate categories, thus any engineering failure codes or complaint trending data, would be insignificant. The 54 units serviced all experienced the same failures, the 9 manufactured during 09/2013 and 11/2013 all were in use, when they malfunctioned. Six patients experienced severe nerve and tissue damage Two patients suffered from long term partial paralysis One patient was permanently paralyzed

QUESTIONS? Dennis R. Hock 412 -644 -3394, Ext. #13 Dennis. Hock@fda. hhs. gov
 Dennis hock
Dennis hock Clinical investigator training program
Clinical investigator training program Internal input devices
Internal input devices 2005-1947
2005-1947 Dee ward hock
Dee ward hock Dee ward hock
Dee ward hock Jürgen hock
Jürgen hock Kai hock
Kai hock Phang swee kim v beh i hock
Phang swee kim v beh i hock Hock aun teh
Hock aun teh Ivd 4 pint
Ivd 4 pint Dee hock
Dee hock Www.sharedinvestigator.com www.sharedinvestigator.com
Www.sharedinvestigator.com www.sharedinvestigator.com Did investigator assign exposures
Did investigator assign exposures Rough sketch vs final sketch crime scene
Rough sketch vs final sketch crime scene Diversion investigator
Diversion investigator Private investigator craigslist
Private investigator craigslist Cps special investigator
Cps special investigator Bio-rad
Bio-rad Statistics investigation
Statistics investigation Child family investigator
Child family investigator Early stage investigator nih
Early stage investigator nih X-ways investigator
X-ways investigator Userra vets investigator
Userra vets investigator Investigator meeting services
Investigator meeting services Invasive species investigator worksheet
Invasive species investigator worksheet Social media forensics
Social media forensics Invasive species investigator worksheet
Invasive species investigator worksheet Investigator responsibilities gcp
Investigator responsibilities gcp Investigator's brochure
Investigator's brochure Gmo investigator kit
Gmo investigator kit Velo cardio facial syndrome
Velo cardio facial syndrome Ontario ombudsman investigator
Ontario ombudsman investigator Swf investigator
Swf investigator Esrc new investigator grant success rate
Esrc new investigator grant success rate Chin interactive investigator
Chin interactive investigator Office of the correctional investigator
Office of the correctional investigator