Shared Investigator Platform Overview Last update 15 May





- Slides: 11

Shared Investigator Platform Overview Last update: 15 May 2020

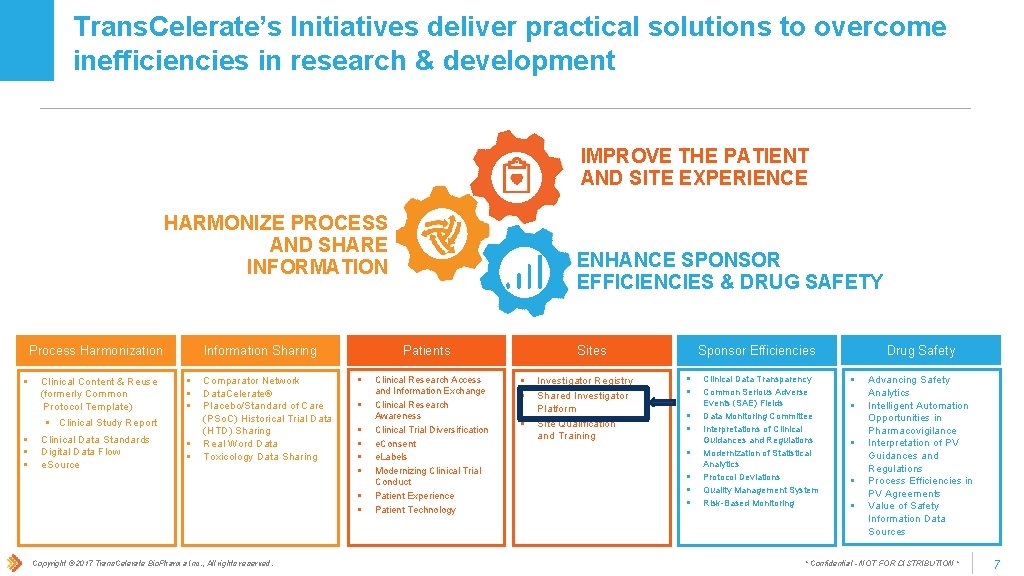
Trans. Celerate’s Initiatives deliver practical solutions to overcome inefficiencies in research & development IMPROVE THE PATIENT AND SITE EXPERIENCE HARMONIZE PROCESS AND SHARE INFORMATION Process Harmonization § § Clinical Content & Reuse (formerly Common Protocol Template) § Clinical Study Report Clinical Data Standards Digital Data Flow e. Source Information Sharing § § § Comparator Network Data. Celerate® Placebo/Standard of Care (PSo. C) Historical Trial Data (HTD) Sharing Real Word Data Toxicology Data Sharing Patients § § § § Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. ENHANCE SPONSOR EFFICIENCIES & DRUG SAFETY Clinical Research Access and Information Exchange Clinical Research Awareness Clinical Trial Diversification e. Consent e. Labels Modernizing Clinical Trial Conduct Patient Experience Patient Technology Sites § § § Investigator Registry Shared Investigator Platform Site Qualification and Training Drug Safety Sponsor Efficiencies § § § § Clinical Data Transparency Common Serious Adverse Events (SAE) Fields Data Monitoring Committee Interpretations of Clinical Guidances and Regulations Modernization of Statistical Analytics Protocol Deviations Quality Management System Risk-Based Monitoring § § § Advancing Safety Analytics Intelligent Automation Opportunities in Pharmacovigilance Interpretation of PV Guidances and Regulations Process Efficiencies in PV Agreements Value of Safety Information Data Sources * Confidential - NOT FOR DISTRIBUTION * 7

Shared Investigator Platform UNMET NEED Clinical trial sites must use many different websites, each requiring unique login credentials to perform clinical trial responsibilities and communicate with their Sponsors. Site staff repeatedly prepare and provide the same information to each of their Sponsors. This is time consuming, cumbersome, and often difficult. What is the Shared Investigator Platform? OBJECTIVE Reduce the burden on investigative sites by providing them with a central point of access, harmonized content and services, and streamlined interaction with participating clinical trial Sponsors. BENEFITS Revolutionized site-sponsor relationship by enabling a single experience through an easy-touse portal, ultimately allowing investigators to spend more time on patients Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *

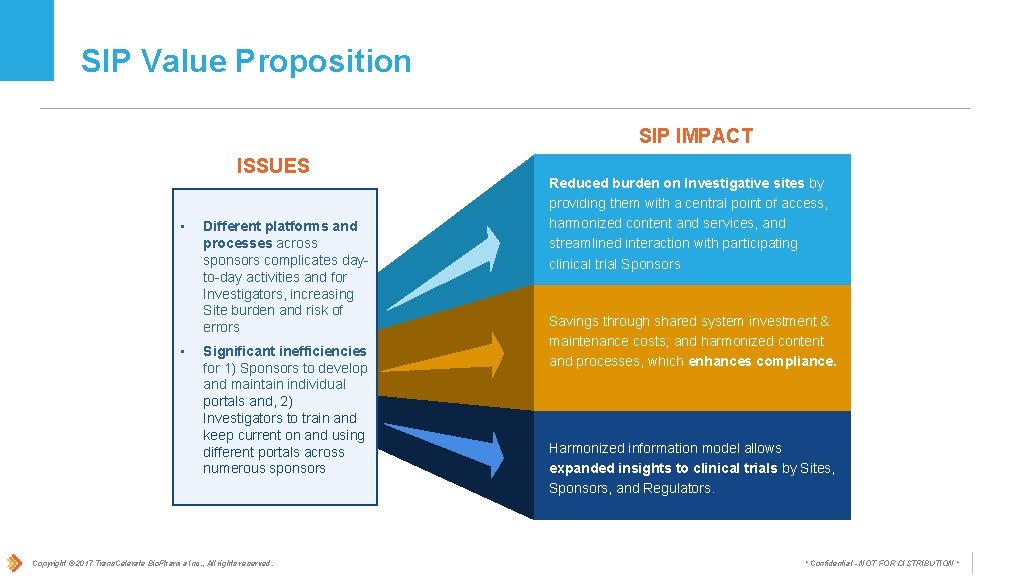
SIP Value Proposition SIP IMPACT ISSUES • • Different platforms and processes across sponsors complicates dayto-day activities and for Investigators, increasing Site burden and risk of errors Significant inefficiencies for 1) Sponsors to develop and maintain individual portals and, 2) Investigators to train and keep current on and using different portals across numerous sponsors Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. Reduced burden on Investigative sites by providing them with a central point of access, harmonized content and services, and streamlined interaction with participating clinical trial Sponsors Savings through shared system investment & maintenance costs; and harmonized content and processes, which enhances compliance. Harmonized information model allows expanded insights to clinical trials by Sites, Sponsors, and Regulators. * Confidential - NOT FOR DISTRIBUTION *

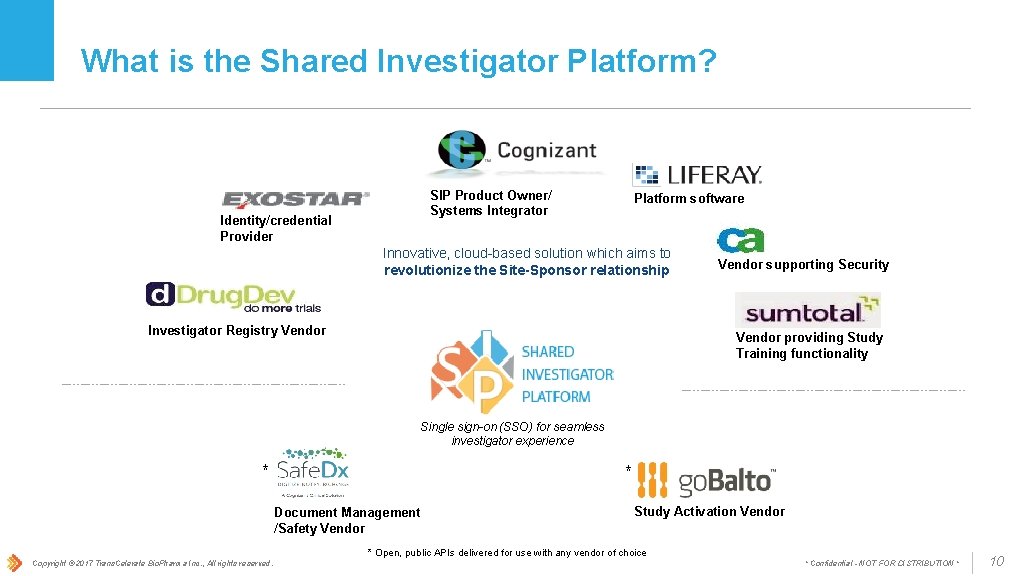
What is the Shared Investigator Platform? SIP Product Owner/ Systems Integrator Identity/credential Provider Platform software Innovative, cloud-based solution which aims to revolutionize the Site-Sponsor relationship Investigator Registry Vendor supporting Security Vendor providing Study Training functionality Single sign-on (SSO) for seamless investigator experience * * Document Management /Safety Vendor Study Activation Vendor * Open, public APIs delivered for use with any vendor of choice Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION * 10

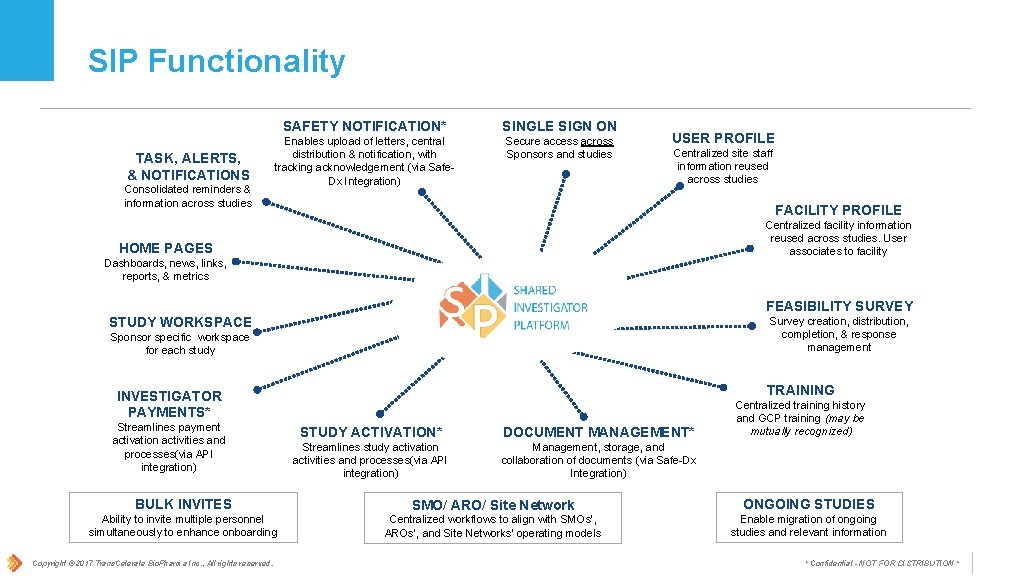
SIP Functionality TASK, ALERTS, & NOTIFICATIONS Consolidated reminders & information across studies SAFETY NOTIFICATION* SINGLE SIGN ON Enables upload of letters, central distribution & notification, with tracking acknowledgement (via Safe. Dx Integration) Secure access across Sponsors and studies USER PROFILE Centralized site staff information reused across studies FACILITY PROFILE Centralized facility information reused across studies. User associates to facility HOME PAGES Dashboards, news, links, reports, & metrics FEASIBILITY SURVEY STUDY WORKSPACE Survey creation, distribution, completion, & response management Sponsor specific workspace for each study TRAINING INVESTIGATOR PAYMENTS* Streamlines payment activation activities and processes(via API integration) STUDY ACTIVATION* DOCUMENT MANAGEMENT* Streamlines study activation activities and processes(via API integration) Management, storage, and collaboration of documents (via Safe-Dx Integration) Centralized training history and GCP training (may be mutually recognized) BULK INVITES SMO/ ARO/ Site Network ONGOING STUDIES Ability to invite multiple personnel simultaneously to enhance onboarding Centralized workflows to align with SMOs’, AROs’, and Site Networks’ operating models Enable migration of ongoing studies and relevant information Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *

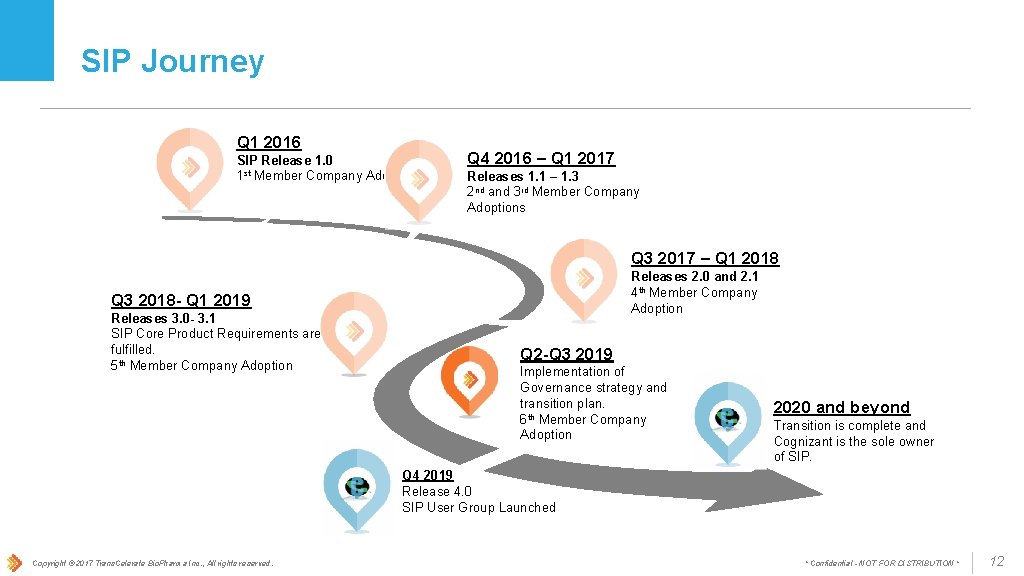
SIP Journey Q 1 2016 SIP Release 1. 0 1 st Member Company Adoption Q 4 2016 – Q 1 2017 Releases 1. 1 – 1. 3 2 nd and 3 rd Member Company Adoptions Q 3 2017 – Q 1 2018 Releases 2. 0 and 2. 1 4 th Member Company Adoption Q 3 2018 - Q 1 2019 Releases 3. 0 - 3. 1 SIP Core Product Requirements are fulfilled. 5 th Member Company Adoption Q 2 -Q 3 2019 Implementation of Governance strategy and transition plan. 6 th Member Company Adoption 2020 and beyond Transition is complete and Cognizant is the sole owner of SIP. Q 4 2019 Release 4. 0 SIP User Group Launched Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION * 12

Resources Press Releases / Whitepapers Videos • Trans. Celerate SIP Launch Press Release • Site Capabilities Made Simple with SIP • Cognizant Press Release • Study Management Made Simple with SIP • Cognizant Whitepaper • Shared Investigator Platform: From Concept to Reality • SIP Fast Facts • Sites Subcommittee Overview Change Management Aides • SIP Initiative Assets Page • Shared Investigator Platform Home Page Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *

Thank You! Visit us, for more information: www. Trans. Celerate. Bio. Pharma. Inc. com Watch our “About Us” Video Sign up for our Newsletter, Accelerate to Innovate @Trans. Celerate Bio. Pharma Inc.

Appendix

SIP Technology Vendors & Components • Cognizant: Product Owner, Platform Owner and System Integrator • Computer Associates: Provides software components for security testing and verifies security during test phases as necessary • Drug. Dev: Manages Investigator Registry (IR), where user/facility data (including audit trails associated with these functions) are stored • Exostar Federation: Enables single sign-on to multiple sponsor systems • Liferay: Collection of design and development tools for web-based applications; SIP uses configured and customized elements from Liferay • Sum. Total: Provides Learning Management System (LMS); Sum. Total is a Saa. S software package customized and seamlessly integrated into SIP • Safe-Dx: Safe-Dx is a Document Management Vendor that enables management and collaboration of documents and Safety notifications. Safe-Dx functionality is available through API Integration. • Go. Balto: Go. Balto is a Study Activation Vendor that streamlines study activation processes and activities. Go. Balto functionality is available through API integration. Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *