Enzymes as Tracers As naturally occurring components Enzyme



- Slides: 20

Enzymes as Tracers As naturally occurring components: Enzyme activities may be monitored to assess the state of a biological system. Pure enzymes may also be employed to modify the condition of analytical specimens to make analyses simpler. Or, they may be incorporated into complexes with ligands or binding components to produce tags that can be identified by the generation of unique products.

Endogenous Tracers Enzymes that are unique to a given cell or tissue provide a means to locate those cells or to ascertain the health of the tissue. For example, alkaline phosphatase is found in few cells but is present in high levels in migrating germ cell precursors, allowing them to be located within the tissues of the developing embryo. Creatine kinase is found in muscle & is normally low in serum unless muscle damage has occurred. Active caspases are only in apoptotic cells.

Endogenous Tracers (cont. ) Enzymes in tissues may also complicate analyses that attempt to use pure enzymes as tracer tags. If samples to be analyzed contain an enzyme with similar properties to the tracer, false positives will arise. Controls lacking tracer enzyme are always needed & protocols should take care that assay conditions optimize specificity of the tracer enzyme activity, e. g. , lack of similar enzymes in samples, p. H, salt, temperature, substrate conditions favoring the tracer.

Pure Tracer Enzymes favored include those with: High turnover numbers Low Km for substrate, high Km for product High Ki Storage stability Ease of detection Low cost/ ease of isolation of pure enzyme Absent in samples Compatible with assay conditions

Pure Tracer Enzymes (cont. ) Horseradish peroxidase Alkaline phosphatase β-Galactosidase Urease Glucose oxidase Acetylcholinesterase Lysozyme Glucose-6 -phosphate dehydrogenase Luciferase

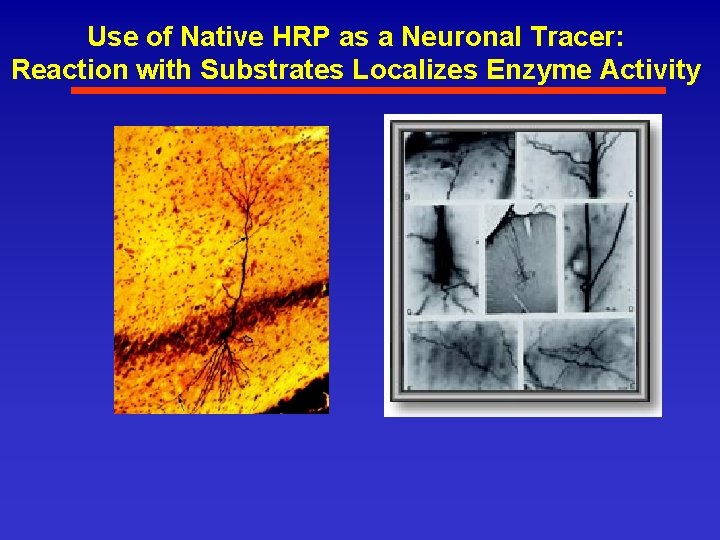
Use of Native HRP as a Neuronal Tracer: Reaction with Substrates Localizes Enzyme Activity

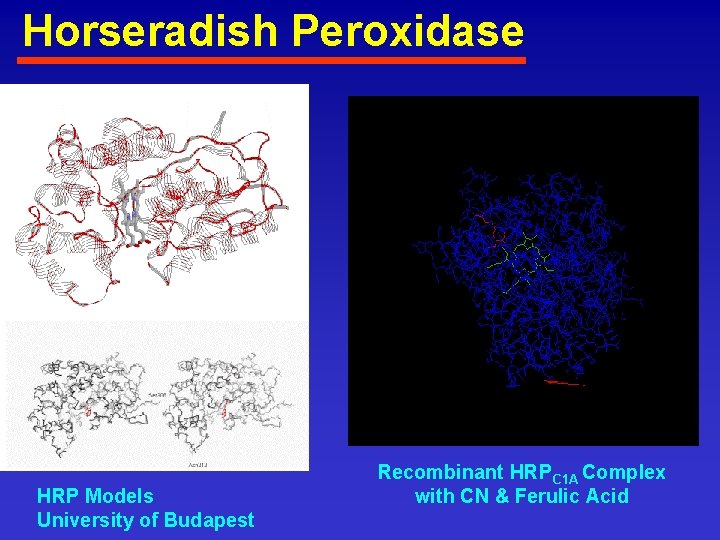
Horseradish Peroxidase HRP Models University of Budapest Recombinant HRPC 1 A Complex with CN & Ferulic Acid

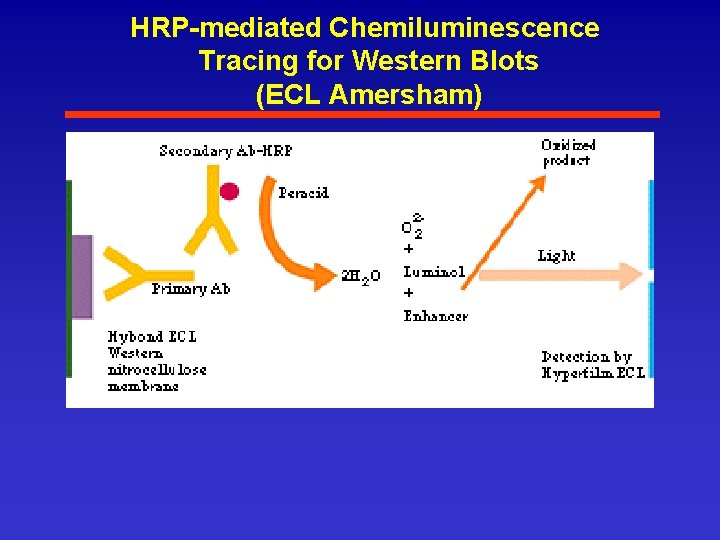
HRP-mediated Chemiluminescence Tracing for Western Blots (ECL Amersham)

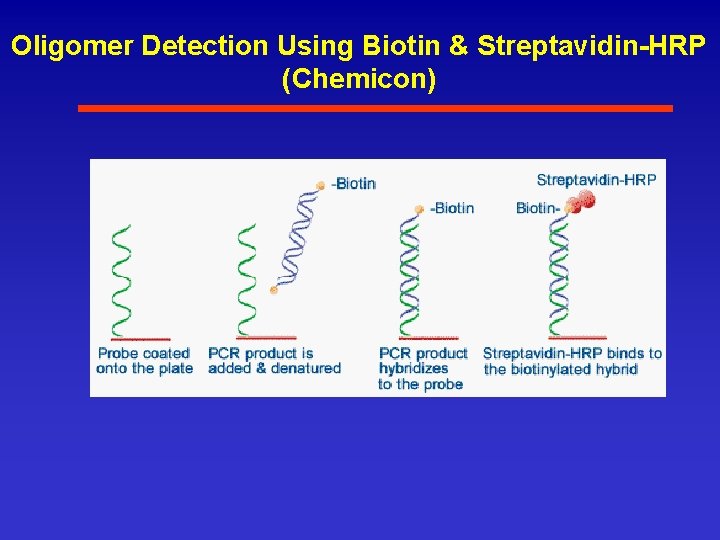
Oligomer Detection Using Biotin & Streptavidin-HRP (Chemicon)

Alkaline Phosphatase University of Oklahoma Health Science Center Clinical Lab PPT slide #59

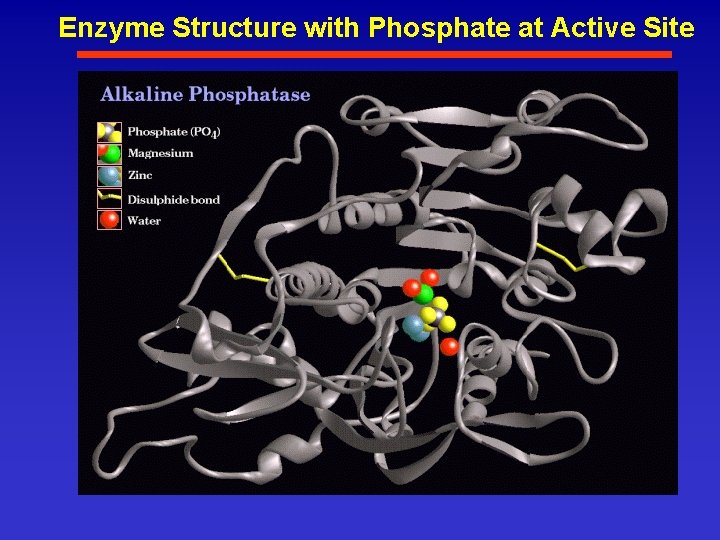
Enzyme Structure with Phosphate at Active Site

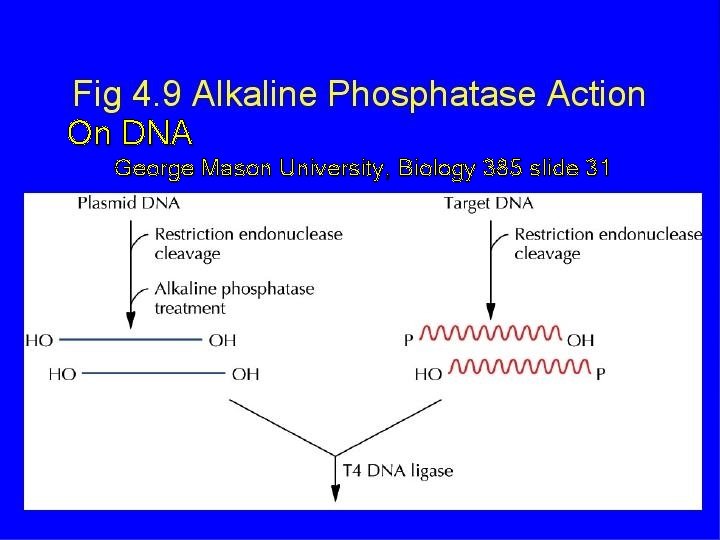
On DNA George Mason University, Biology 385 slide 31

Alkaline Phosphatase Chemilumenescence on Nylon (University of Bologna)


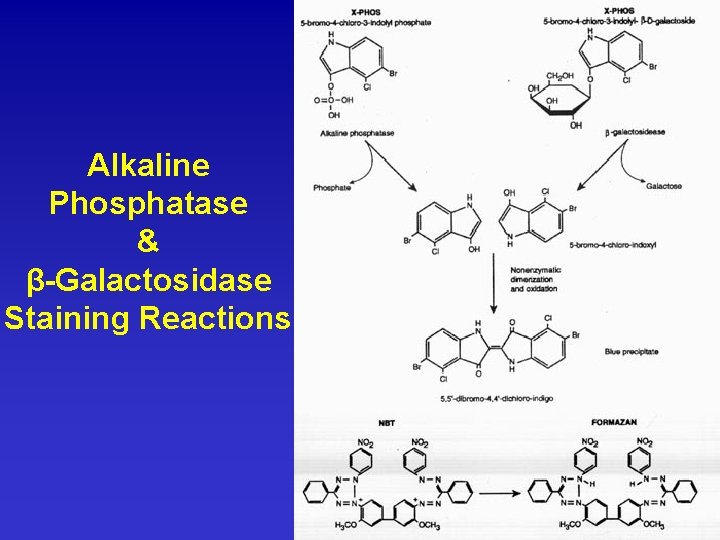
Alkaline Phosphatase & β-Galactosidase Staining Reactions

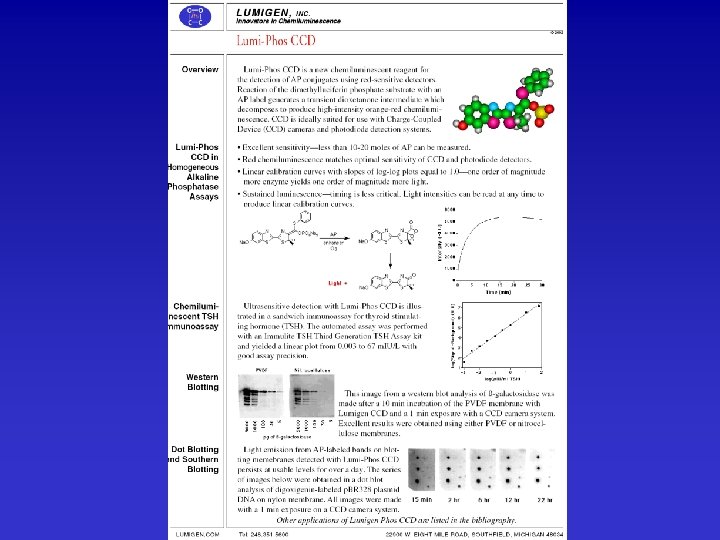
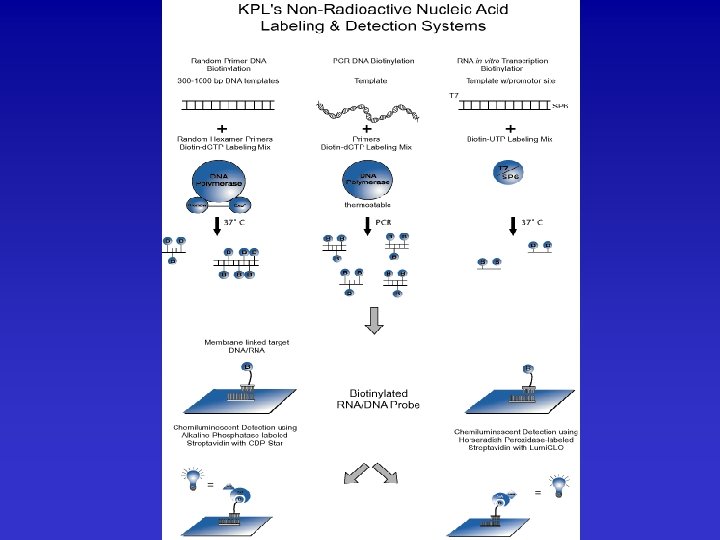
There is substantial flexibility available in production of soluble, insoluble, chromogenic, fluorescent, or luminescent products from either alkaline phosphatase or horseradish peroxidase. The choice is largely based on desired data, limit of detection required, available instrumentation, ancillary reagents, and prior experience. Substrates are available from Sigma/Aldrich Chemicals, In Vitrogen, Anaspec, Pierce Chemical, and a number of other suppliers. For some idea of this range see: Sigma. Alk. Phos&HRPSubstrates. pdf

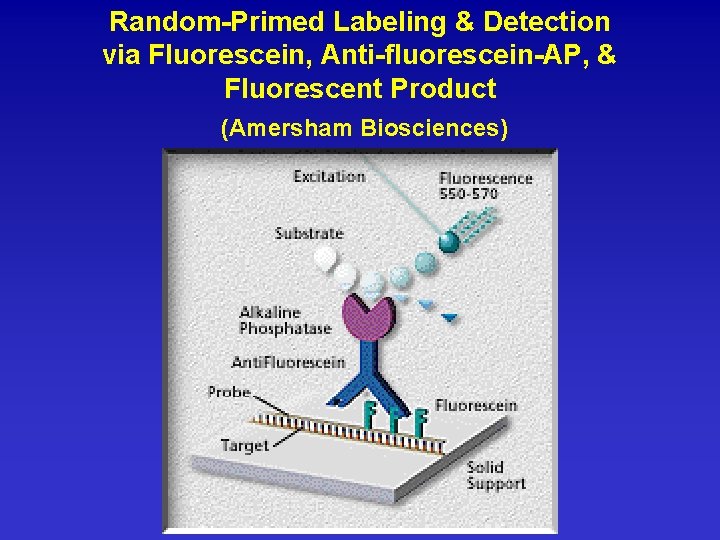
Random-Primed Labeling & Detection via Fluorescein, Anti-fluorescein-AP, & Fluorescent Product (Amersham Biosciences)

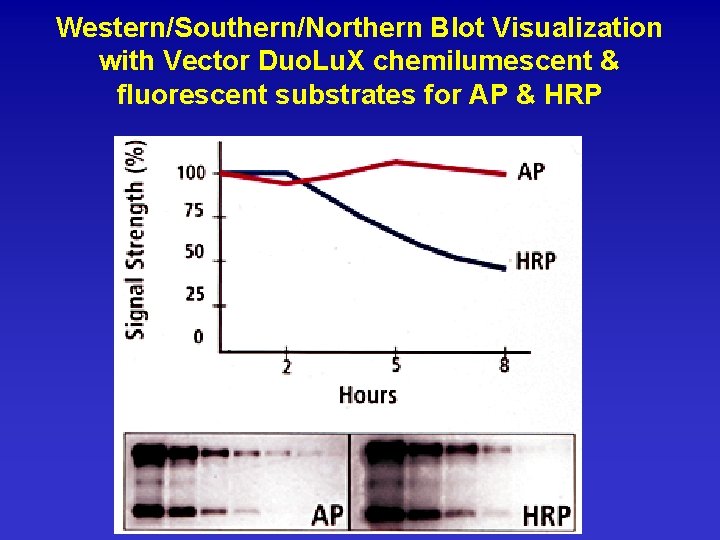
Western/Southern/Northern Blot Visualization with Vector Duo. Lu. X chemilumescent & fluorescent substrates for AP & HRP


Some Cautions: Use of HRP or AP often requires pretreatment of specimens or solutions to remove endogenous enzyme activity or related enzyme activities. Be aware that the enzymes can be inhibited by their products or by inhibitors, e. g. , acid p. H & phosphate buffers block AP activity while perservatives such as azide block HRP activity. Enzyme handling is also crucial: AP solutions can be stored frozen while HRP solutions cannot; while both enzymes like all proteins are sensitive to freezing & thawing, this action is hardest on enzymes with prosthetic groups & those involved in redox reactions; storage with cryoprotectants such as glycerol, ethylene glycol, or DMSO helps considerably.