ENV 4101 Elements of Air Pollution ENV 5105



- Slides: 15

ENV 4101 Elements of Air Pollution ENV 5105 Foundations of Air Pollution Equilibrium & Kinetics Prepared by: Ying Li

Equilibrium & Kinetics • Equilibrium constant • Free energy • Temperature effect • Kinetics • Order of Reactions (1 st, 2 nd, and pseudo 1 st order) • Air Pollution Reactions • Lifetime, Half-life • Temperature effect (Arrenhius Equation)

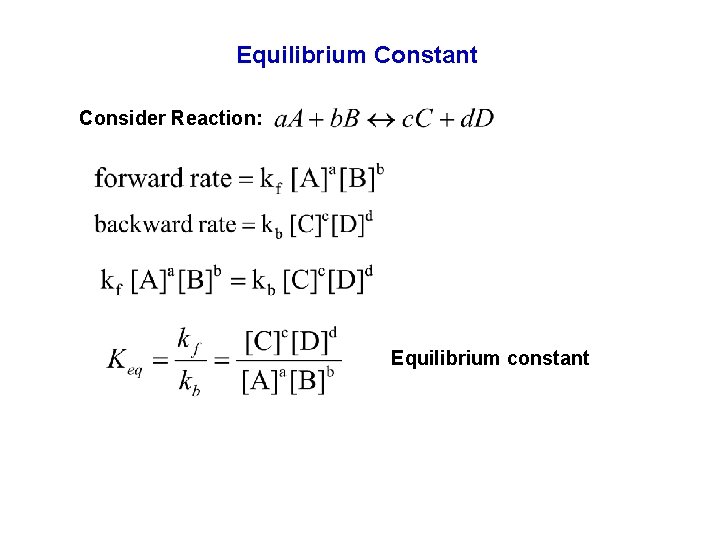
Equilibrium Constant Consider Reaction: Equilibrium constant

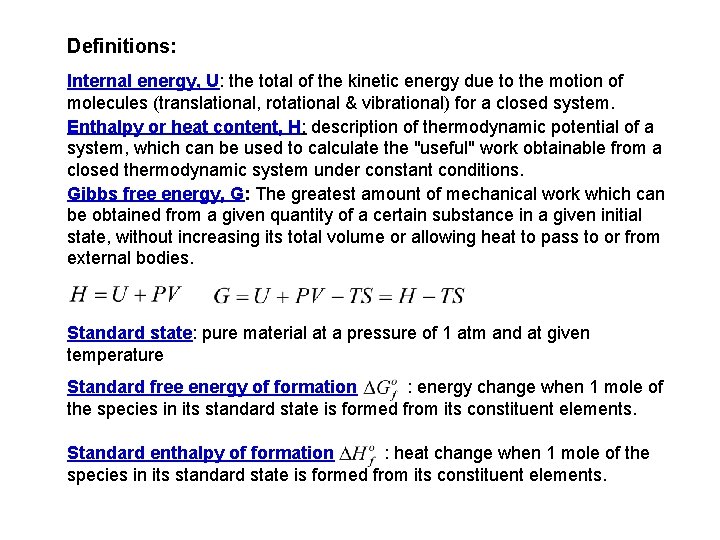
Definitions: Internal energy, U: the total of the kinetic energy due to the motion of molecules (translational, rotational & vibrational) for a closed system. Enthalpy or heat content, H: description of thermodynamic potential of a system, which can be used to calculate the "useful" work obtainable from a closed thermodynamic system under constant conditions. Gibbs free energy, G: The greatest amount of mechanical work which can be obtained from a given quantity of a certain substance in a given initial state, without increasing its total volume or allowing heat to pass to or from external bodies. Standard state: pure material at a pressure of 1 atm and at given temperature Standard free energy of formation : energy change when 1 mole of the species in its standard state is formed from its constituent elements. Standard enthalpy of formation : heat change when 1 mole of the species in its standard state is formed from its constituent elements.

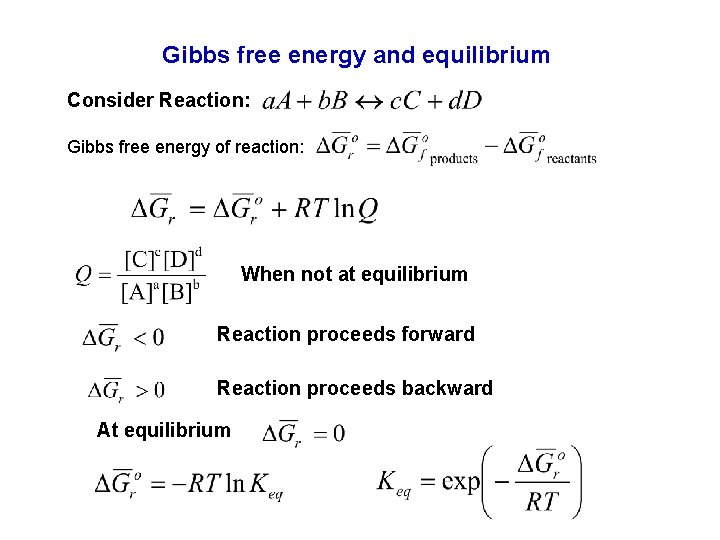
Gibbs free energy and equilibrium Consider Reaction: Gibbs free energy of reaction: When not at equilibrium Reaction proceeds forward Reaction proceeds backward At equilibrium

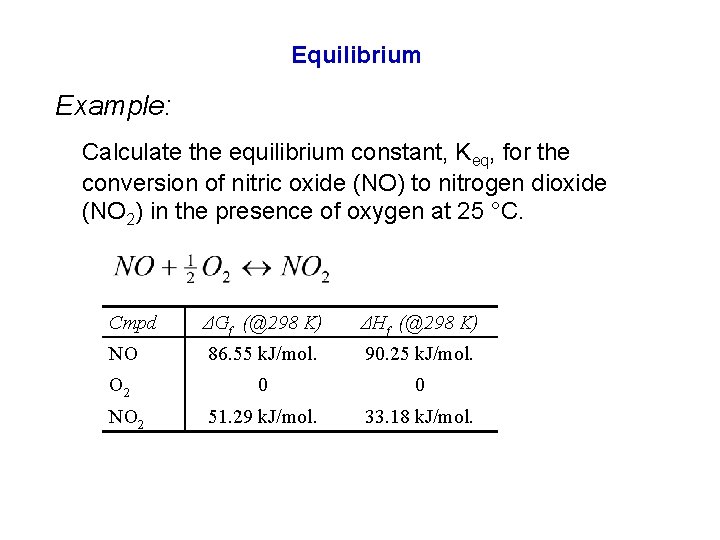
Equilibrium Example: Calculate the equilibrium constant, Keq, for the conversion of nitric oxide (NO) to nitrogen dioxide (NO 2) in the presence of oxygen at 25 °C. Cmpd ΔGf (@298 K) ΔHf (@298 K) NO 86. 55 k. J/mol. 90. 25 k. J/mol. O 2 0 0 51. 29 k. J/mol. 33. 18 k. J/mol. NO 2

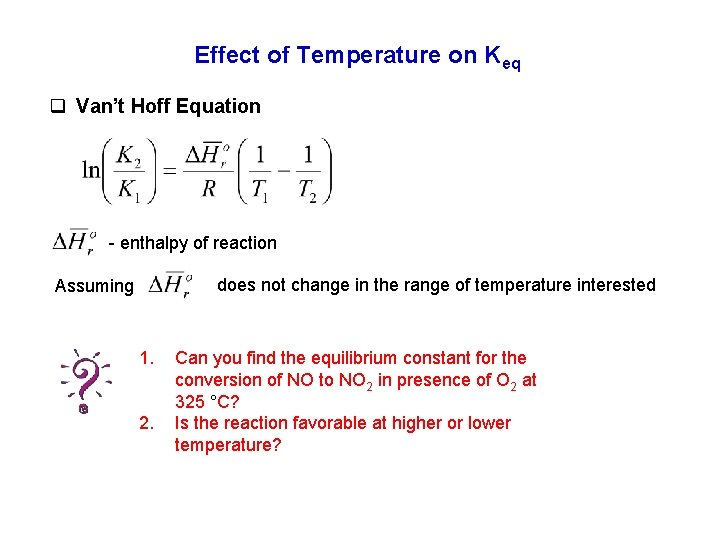
Effect of Temperature on Keq q Van’t Hoff Equation - enthalpy of reaction does not change in the range of temperature interested Assuming 1. 2. Can you find the equilibrium constant for the conversion of NO to NO 2 in presence of O 2 at 325 °C? Is the reaction favorable at higher or lower temperature?

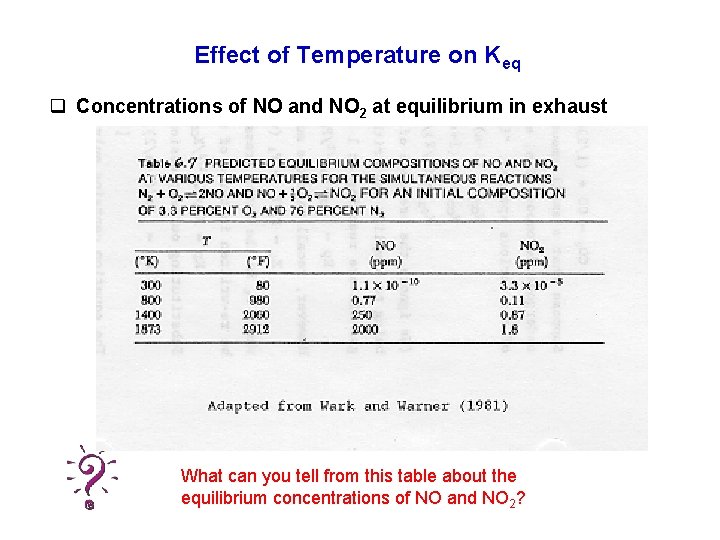
Effect of Temperature on Keq q Concentrations of NO and NO 2 at equilibrium in exhaust What can you tell from this table about the equilibrium concentrations of NO and NO 2?

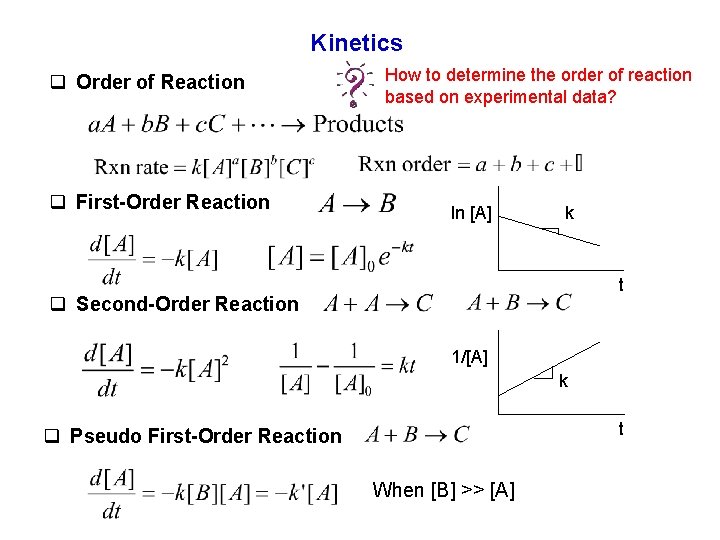
Kinetics q Order of Reaction q First-Order Reaction How to determine the order of reaction based on experimental data? ln [A] k t q Second-Order Reaction 1/[A] k t q Pseudo First-Order Reaction When [B] >> [A]

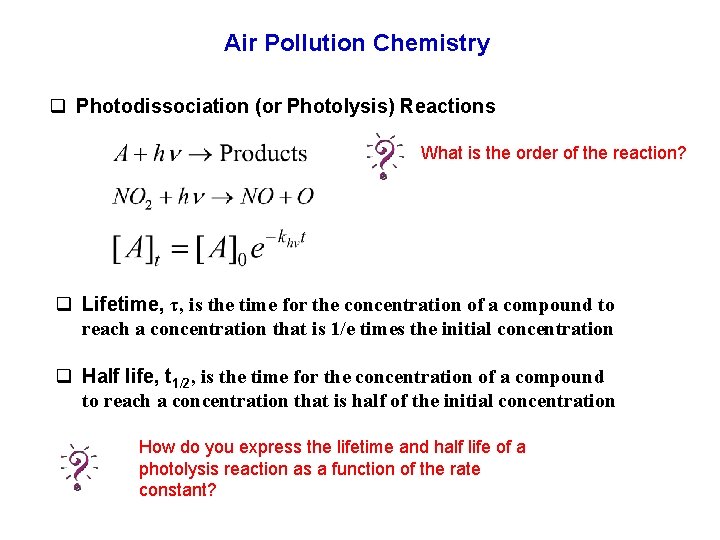
Air Pollution Chemistry q Photodissociation (or Photolysis) Reactions What is the order of the reaction? q Lifetime, τ, is the time for the concentration of a compound to reach a concentration that is 1/e times the initial concentration q Half life, t 1/2, is the time for the concentration of a compound to reach a concentration that is half of the initial concentration How do you express the lifetime and half life of a photolysis reaction as a function of the rate constant?

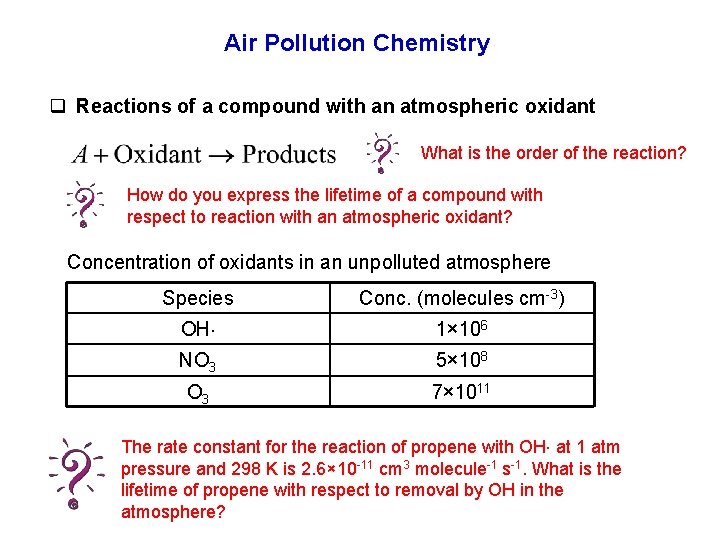
Air Pollution Chemistry q Reactions of a compound with an atmospheric oxidant What is the order of the reaction? How do you express the lifetime of a compound with respect to reaction with an atmospheric oxidant? Concentration of oxidants in an unpolluted atmosphere Species Conc. (molecules cm-3) OH 1× 106 NO 3 5× 108 O 3 7× 1011 The rate constant for the reaction of propene with OH at 1 atm pressure and 298 K is 2. 6× 10 -11 cm 3 molecule-1 s-1. What is the lifetime of propene with respect to removal by OH in the atmosphere?

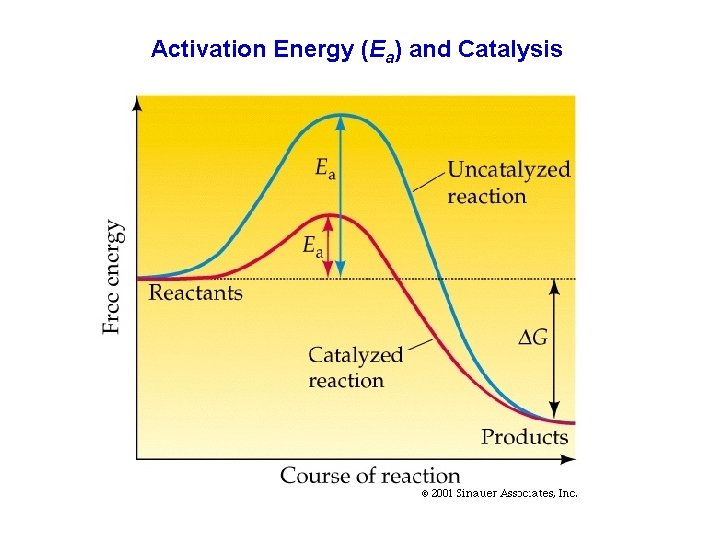
Activation Energy (Ea) and Catalysis

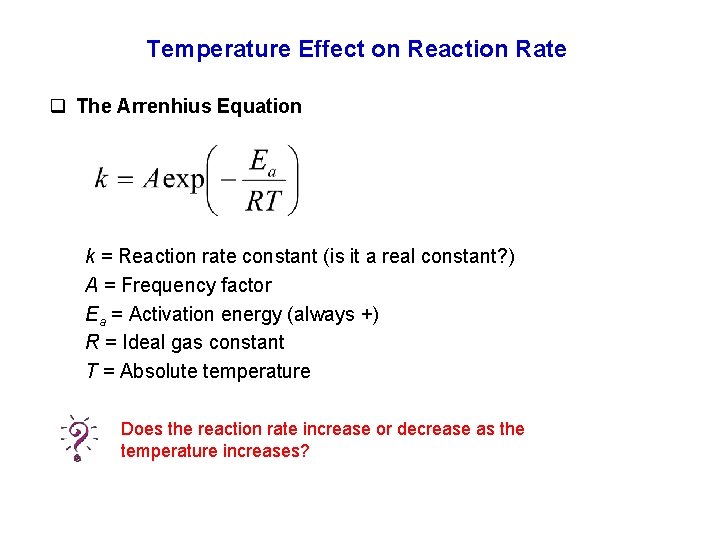
Temperature Effect on Reaction Rate q The Arrenhius Equation k = Reaction rate constant (is it a real constant? ) A = Frequency factor Ea = Activation energy (always +) R = Ideal gas constant T = Absolute temperature Does the reaction rate increase or decrease as the temperature increases?

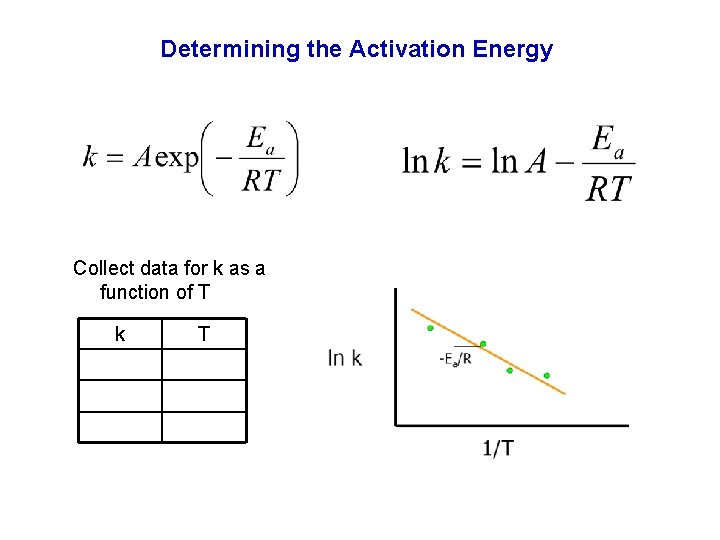
Determining the Activation Energy Collect data for k as a function of T k T

Quick Reflections • Equilibrium constant • Free energy • Temperature effect • Kinetics • Order of Reactions (1 st, 2 nd, and pseudo 1 st order) • Air Pollution Reactions • Lifetime, Halflife • Temperature effect (Arrenhius Equation)