Energy In Chemical Reactions Exothermic vs Endothermic Reactions



- Slides: 10

Energy In Chemical Reactions Exothermic vs. Endothermic Reactions

How Do Chemical Reactions Create Heat energy? �Consider the combustion of gasoline (octane) 2 C 8 H 18 +25 O 2 16 CO 2 +18 H 2 O �Potential Energy = Stored energy • Potential energy is stored in the bonds of the reactants and the products • When bonds are broken, the energy is available • When the products form bonds, some energy is used in these bonds • The excess energy is released as heat

Law of Conservation of Energy �Energy is not lost or gained in a chemical reaction � In a chemical reaction, stored chemical energy (potential energy) is transferred to kinetic energy

System vs. Surroundings �System: • the part of the universe on which we focus attention • The reactants and products in a reaction �Surroundings: • Everything else in universe • Air in room and anything else other than reactants and products

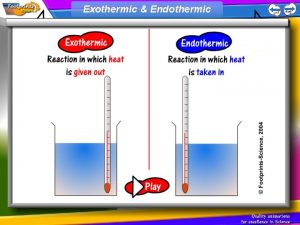
Exothermic Reactions �Results in the evolution of heat �Energy flows out of the system into the surroundings �Energy is a product �Some of the potential energy stored in the chemical bonds is converted to thermal or light energy

Example of an Exothermic Reaction �Combustion of Natural Gas (methane) CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 20(g) +energy

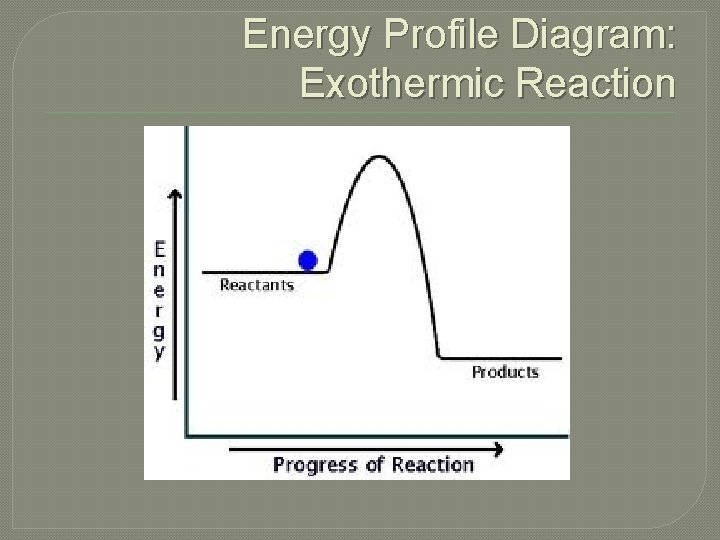
Energy Profile Diagram: Exothermic Reaction

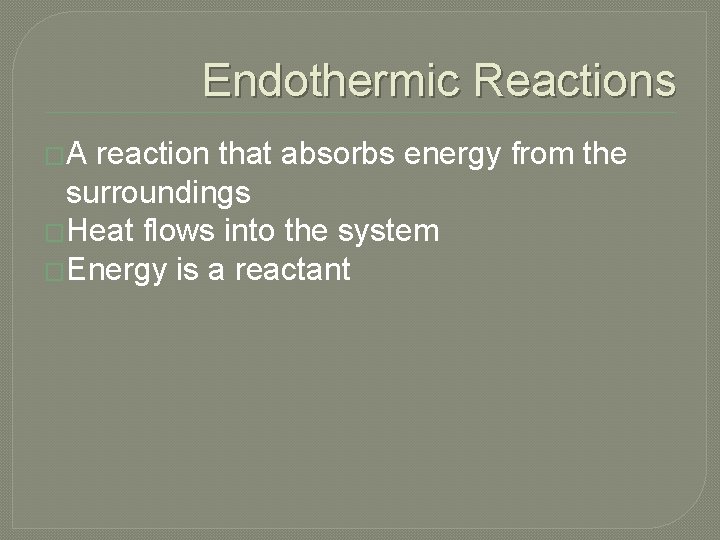
Endothermic Reactions �A reaction that absorbs energy from the surroundings �Heat flows into the system �Energy is a reactant

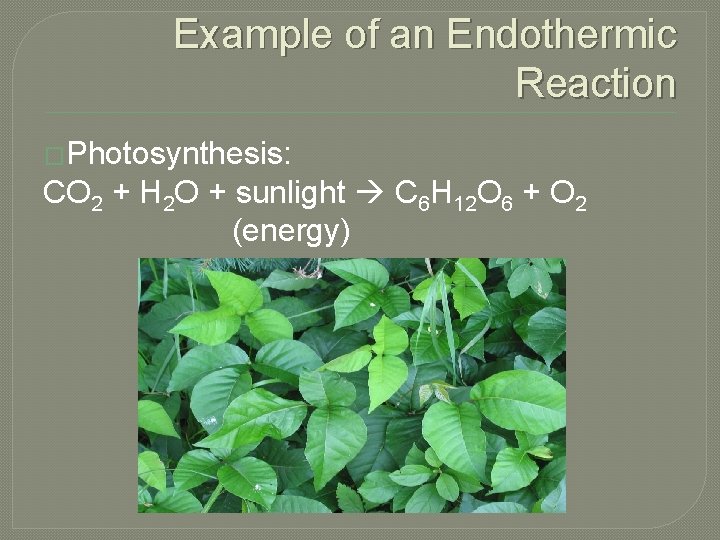
Example of an Endothermic Reaction �Photosynthesis: CO 2 + H 2 O + sunlight C 6 H 12 O 6 + O 2 (energy)

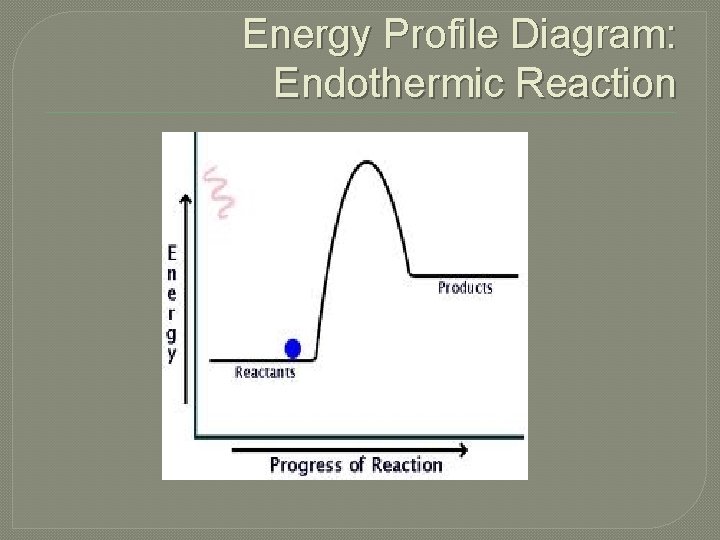
Energy Profile Diagram: Endothermic Reaction
 Endo exo thermic
Endo exo thermic Endothermic vs exothermic
Endothermic vs exothermic Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Is vaporization endothermic or exothermic
Is vaporization endothermic or exothermic Exothermic vs endothermic
Exothermic vs endothermic Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic Exothermic v endothermic
Exothermic v endothermic Heating and cooling curves
Heating and cooling curves Endothermic reaction examples
Endothermic reaction examples Ectotherms
Ectotherms