Energy Exchanges 1 st Law of Thermodynamics Energy



- Slides: 10

Energy Exchanges • 1 st Law of Thermodynamics – Energy cannot be created nor destroyed, energy is transferred from one form to another • 2 nd Law of Thermodynamics – – Every energy transfer makes the universe more disordered Entropy is a measure of this disorder With every energy transfer, heat is lost Amt of useful energy is decreased with every transfer • PROBLEM: living organisms are highly ordered to decrease entropy – Question: Do living organisms violate 2 nd Law?

ANSWER • NO – Living organism is a closed system – Must consider organism and environment – Living organisms… • Maintain highly ordered structure at expense of increased entropy of surroundings • Take in complex high energy molecule, extract energy, release simpler, low energy molecules (CO 2 and H 2 O) and heat to environment

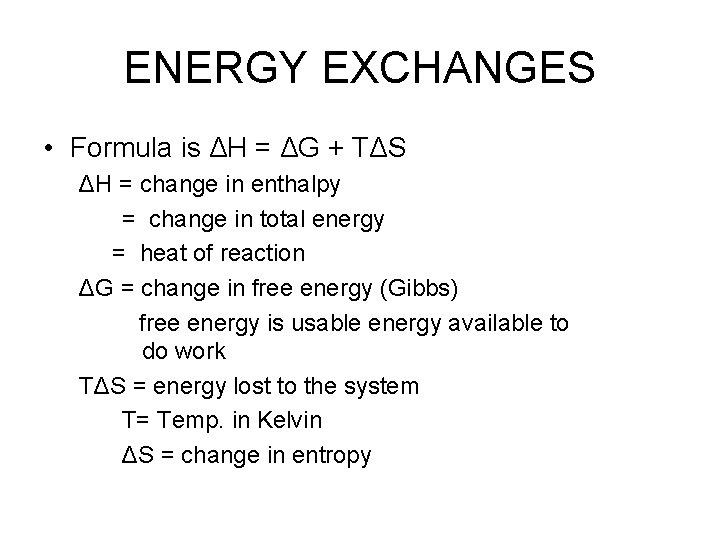
ENERGY EXCHANGES • Formula is ΔH = ΔG + TΔS ΔH = change in enthalpy = change in total energy = heat of reaction ΔG = change in free energy (Gibbs) free energy is usable energy available to do work TΔS = energy lost to the system T= Temp. in Kelvin ΔS = change in entropy

• During every energy exchange (ΔH) some energy is available to do work (ΔG) and some is lost to the system (TΔS) Exergonic and Endergonic

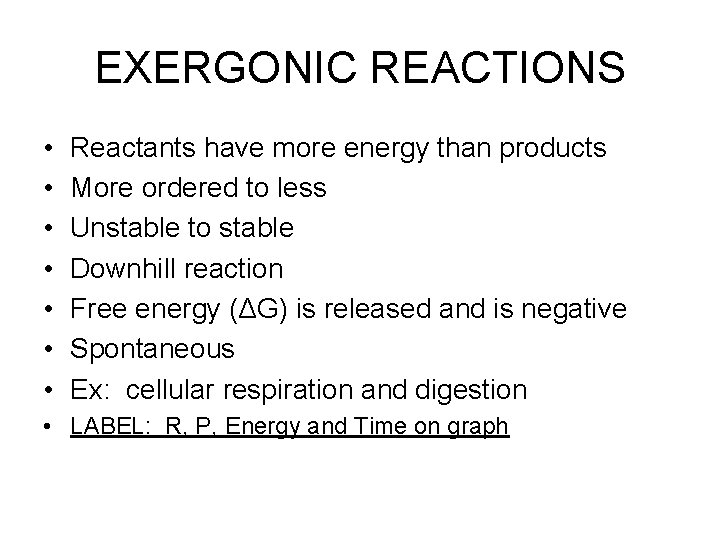
EXERGONIC REACTIONS • • Reactants have more energy than products More ordered to less Unstable to stable Downhill reaction Free energy (ΔG) is released and is negative Spontaneous Ex: cellular respiration and digestion • LABEL: R, P, Energy and Time on graph

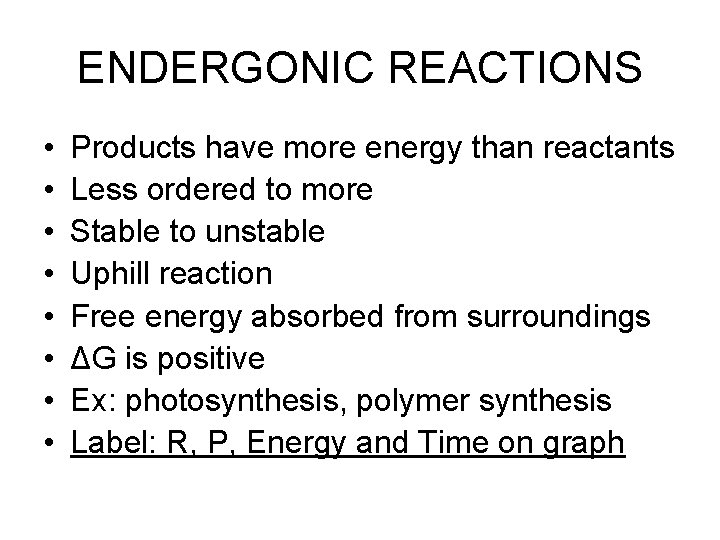
ENDERGONIC REACTIONS • • Products have more energy than reactants Less ordered to more Stable to unstable Uphill reaction Free energy absorbed from surroundings ΔG is positive Ex: photosynthesis, polymer synthesis Label: R, P, Energy and Time on graph

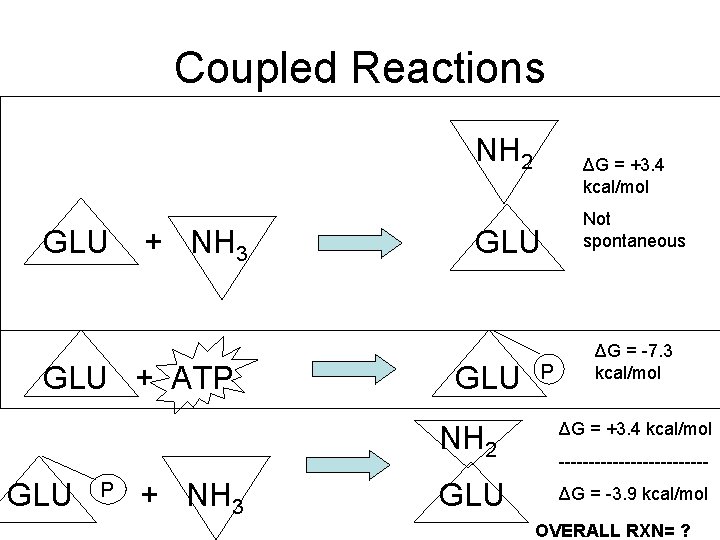
COUPLED REACTIONS • Energy released from exergonic reaction drives endergonic reaction Exergonic ΔG = max work that can be done Endergonic ΔG = min work needed to drive the reaction When these two values are equal – most efficient

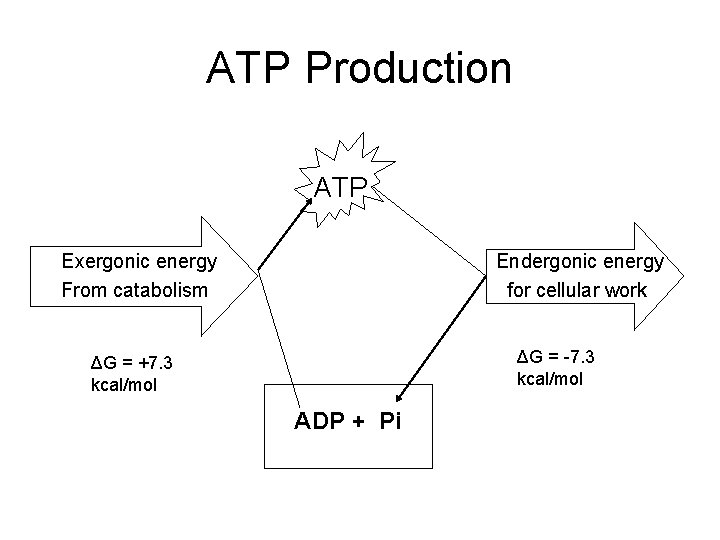
ATP Production ATP Exergonic energy From catabolism Endergonic energy for cellular work ΔG = -7. 3 kcal/mol ΔG = +7. 3 kcal/mol ADP + Pi

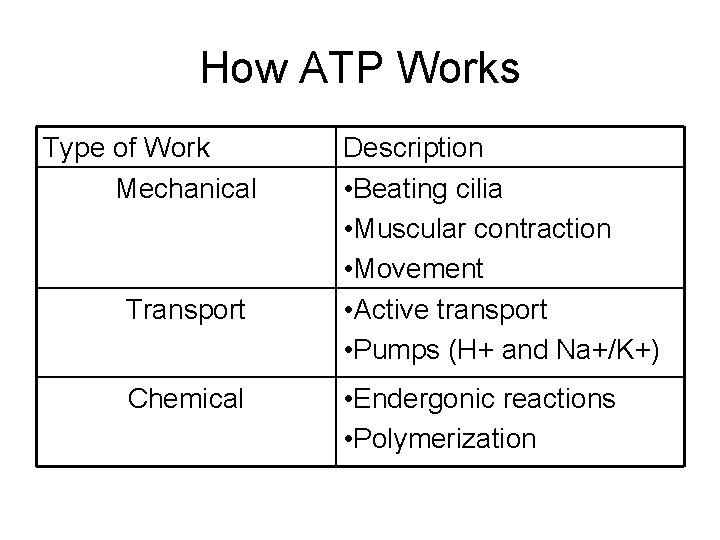
How ATP Works Type of Work Mechanical Transport Chemical Description • Beating cilia • Muscular contraction • Movement • Active transport • Pumps (H+ and Na+/K+) • Endergonic reactions • Polymerization

Coupled Reactions NH 2 GLU + NH 3 GLU + ATP GLU P + NH 3 ΔG = +3. 4 kcal/mol GLU P Not spontaneous ΔG = -7. 3 kcal/mol NH 2 ΔG = +3. 4 kcal/mol GLU ΔG = -3. 9 kcal/mol ------------- OVERALL RXN= ?
 6 exchanges dialogue
6 exchanges dialogue Exchanges over trillion trading volume this
Exchanges over trillion trading volume this The role of internet exchanges and peering
The role of internet exchanges and peering First law of thermodynamics for open system
First law of thermodynamics for open system Oth law of thermodynamics
Oth law of thermodynamics Newtons third law of thermodynamics
Newtons third law of thermodynamics Thermodynamics laws
Thermodynamics laws First law of thermodynamics
First law of thermodynamics Brayton cycle process
Brayton cycle process Formula for entropy change
Formula for entropy change Zeroth law of thermodynamics
Zeroth law of thermodynamics



















