Elements and Their Properties Nonmetals and Metalloids Examples






- Slides: 16

Elements and Their Properties Nonmetals and Metalloids


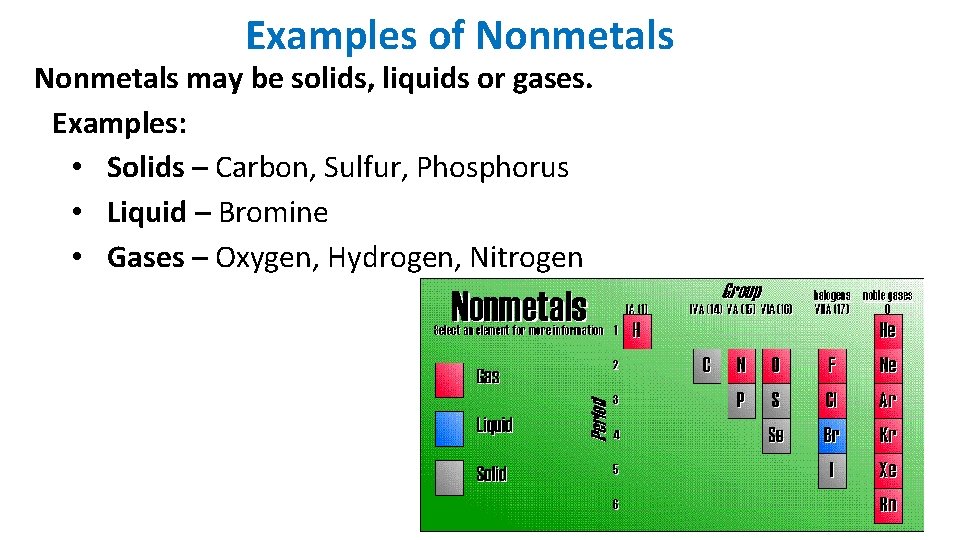
Examples of Nonmetals may be solids, liquids or gases. Examples: • Solids – Carbon, Sulfur, Phosphorus • Liquid – Bromine • Gases – Oxygen, Hydrogen, Nitrogen

Physical Properties of Nonmetals • Nonmetals have a dull luster. Example: Phosphorus

Physical Properties of Nonmetals • Nonmetals are insulators • They do not conduct electricity or heat well. The atoms in nonmetals do not have loose electrons. Therefore, when electricity, or something hot touches a non-metal, the energy does not move quickly through the material. What would you rather stir a hot pot with—a wooden spoon or a metal spoon?

Physical Properties of Nonmetals • Nonmetals are soft (except for diamonds) and brittle Example: Sulfur

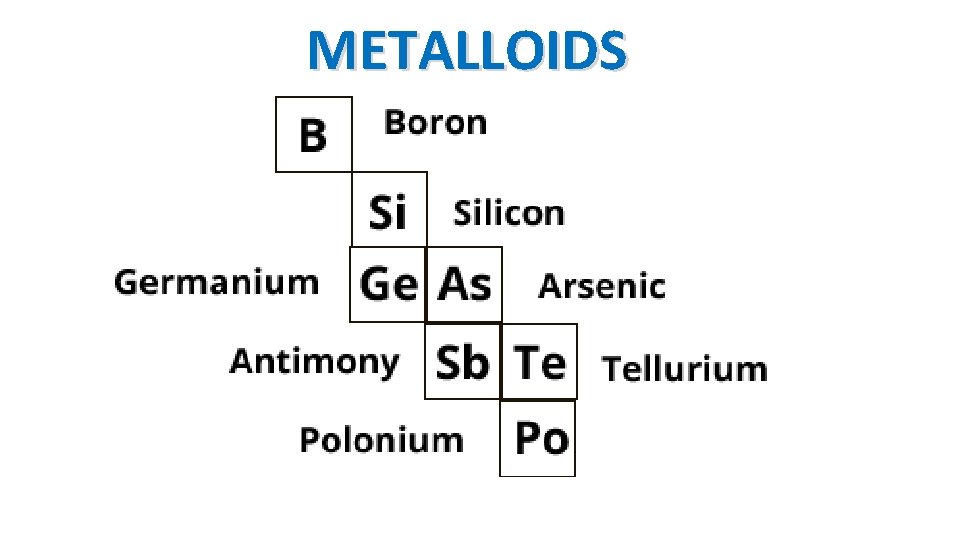
METALLOIDS

Metalloids Physical properties of both metals and non-metals • Some are shiny, some are dull • Somewhat malleable and ductile • Can conduct heat and electricity at a lesser level than metals – known as semiconductors BORON SILICON ARSENIC

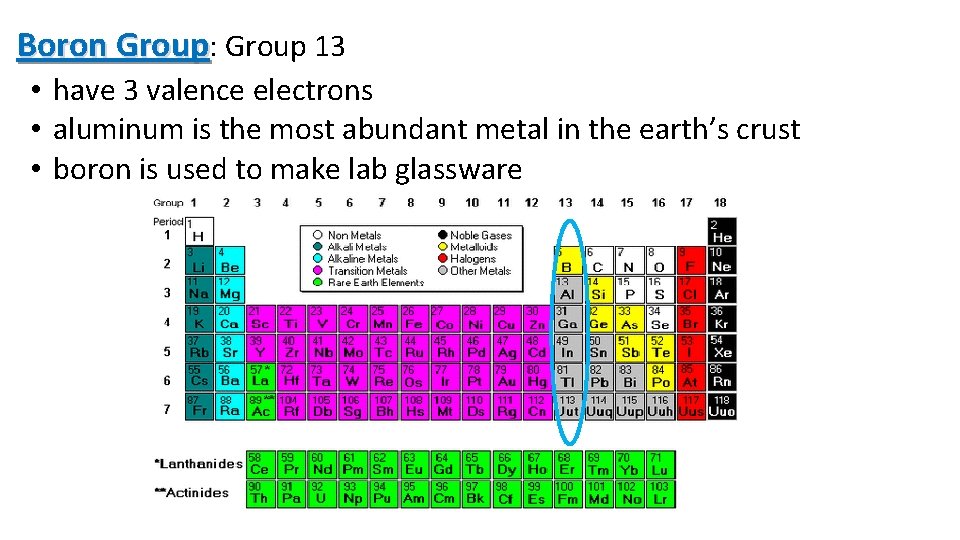
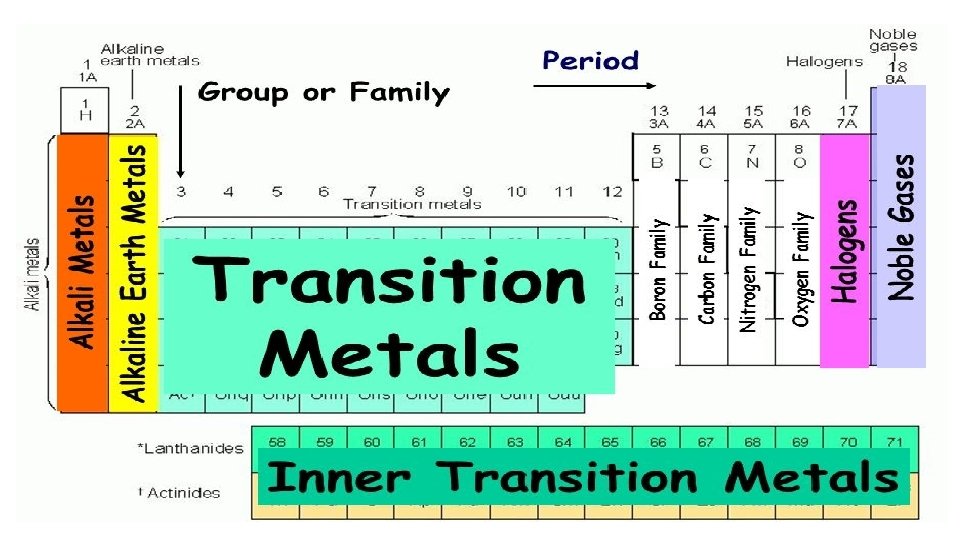
Boron Group: Group 13 • have 3 valence electrons • aluminum is the most abundant metal in the earth’s crust • boron is used to make lab glassware 11

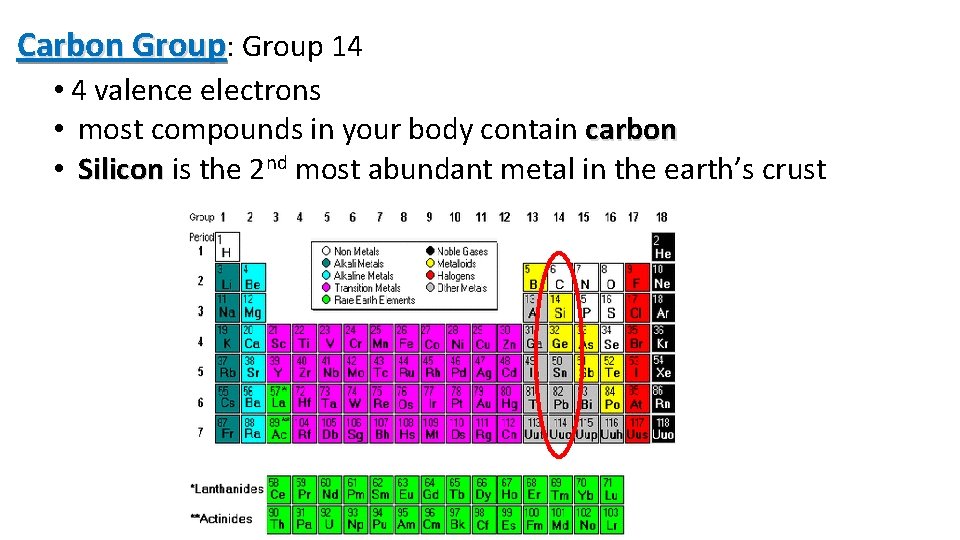
Carbon Group: Group 14 • 4 valence electrons • most compounds in your body contain carbon • Silicon is the 2 nd most abundant metal in the earth’s crust 12

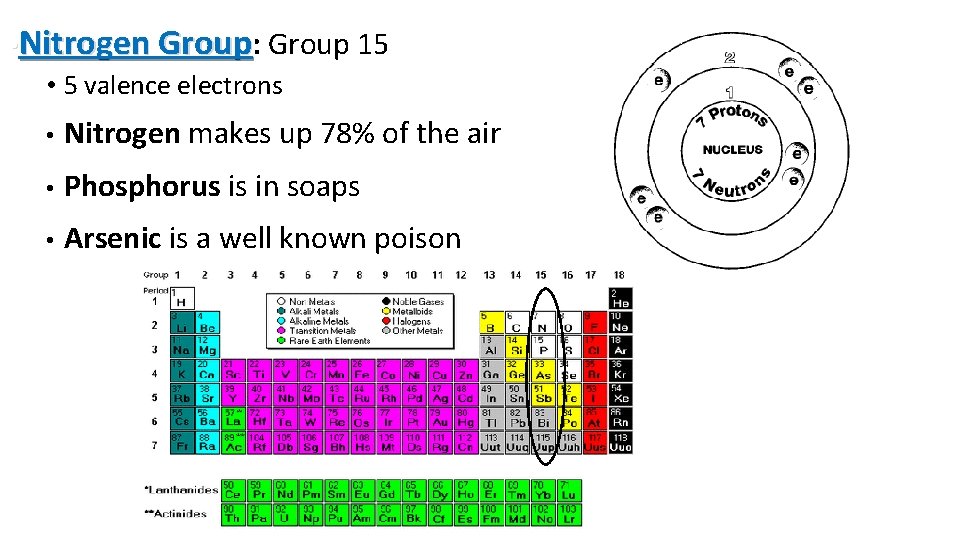
• Nitrogen Group: Group 15 • 5 valence electrons • Nitrogen makes up 78% of the air • Phosphorus is in soaps • Arsenic is a well known poison 14

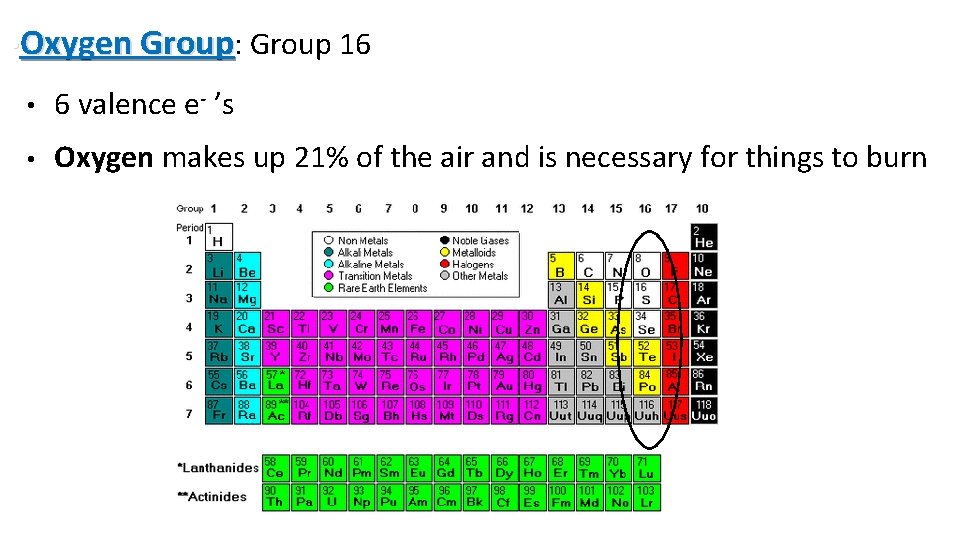
• Oxygen Group: Group 16 • 6 valence e- ’s • Oxygen makes up 21% of the air and is necessary for things to burn 15

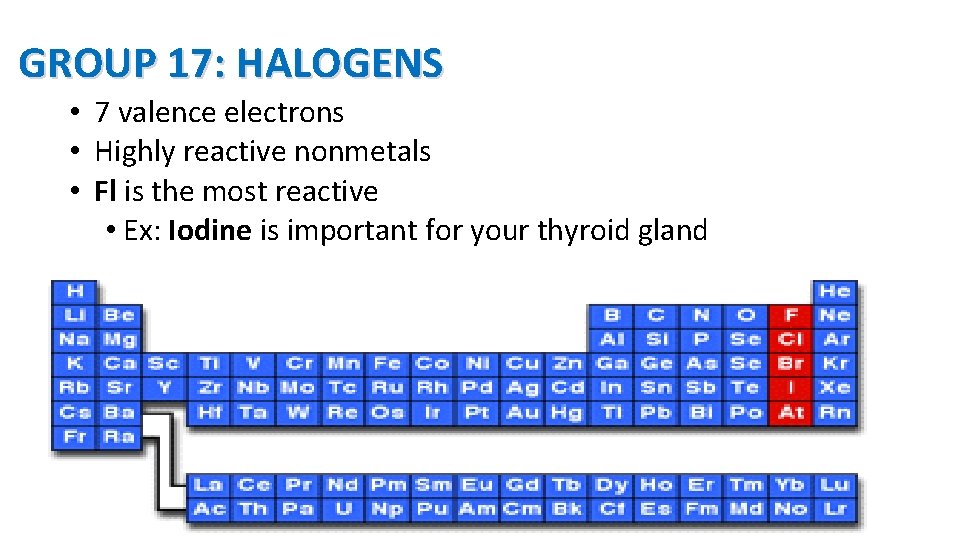
GROUP 17: HALOGENS • 7 valence electrons • Highly reactive nonmetals • Fl is the most reactive • Ex: Iodine is important for your thyroid gland 16

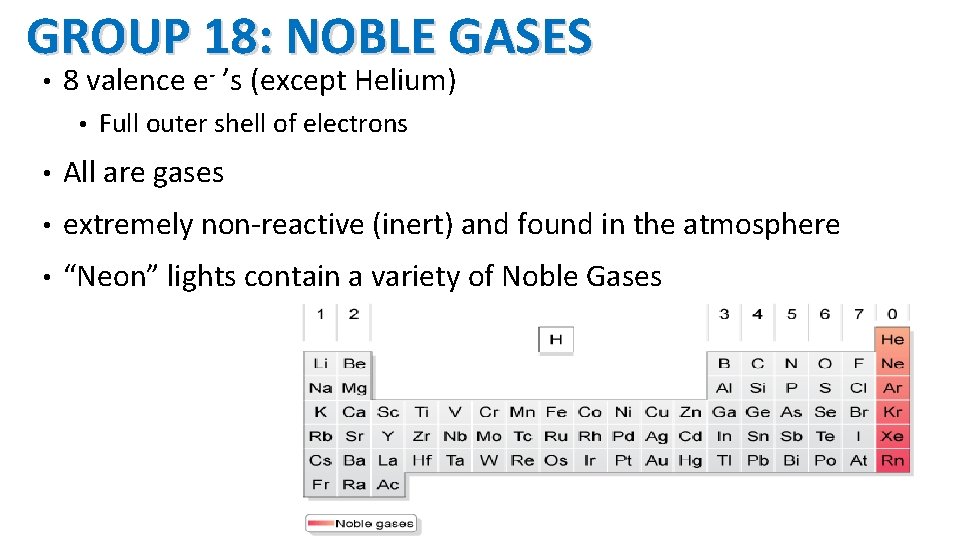
GROUP 18: NOBLE GASES • 8 valence e- ’s (except Helium) • Full outer shell of electrons • All are gases • extremely non-reactive (inert) and found in the atmosphere • “Neon” lights contain a variety of Noble Gases 17

18

Review 1. ____ are columns in the periodic table and there ______ total. 2. _______ are located on the left side of the periodic table and are known for being good conductors. 3. ______ is considered to be the “big daddy” on the periodic table because it is the most reactive element. 4. _____ symbols do not occur naturally in nature. 5. _________ are located between groups 6. 3 -12 and when they form compounds make distinctive colors (ex. Stain glass windows). 7. _____ group is considered to be the least reactive group in the periodic table. 8. Give 2 characteristics of nonmetals.