Electrostatics Electric Charges and Fields Static Electricity u


























- Slides: 49

Electrostatics Electric Charges and Fields

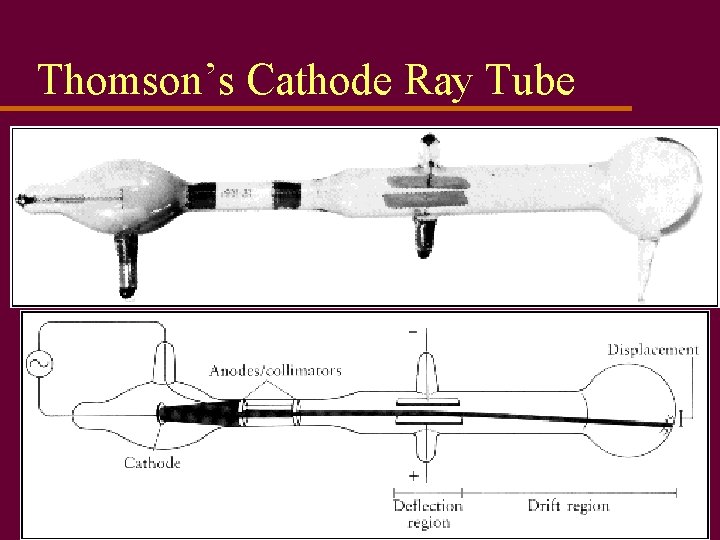
Static Electricity u Called static because charge not pushed by battery, generator, or other emf source u Early experimenters found two types of charge, positive and negative u Ben Franklin (1750’s) made decision which type would be called neg. and pos. u Discovery of electron (Thomson, 1897) showed mobile charge is usually negative

Electric Charges u Enormous amounts of charge exist in all matter but usually no effects are seen due to equal number of positive and negative charges u Electrification occurs when charges are separated u Electric charge is conserved—no charge is created or destroyed, just rearranged

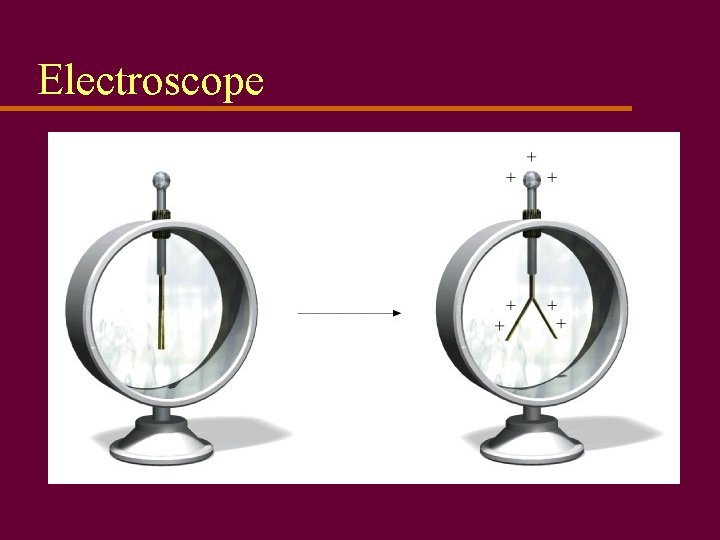
Electric charges u Electrons carry negative charge, protons carry positive charge u Excess electrons makes a negative charge; lack of electrons makes a positive charge u Use electroscope to detect static charge

Measuring Electric Charge u Unit of charge is the coulomb (C), very large amount of charge, equal to 6. 25 x 1018 electrons u The symbol for charge in an equation is q or Q u Electric charge is quantized—the amount of charge is always a multiple of a very small amount

Measuring Electric Charge u Thomson measured the ratio of charge to mass for an electron, but was unable to measure either quantity separately u Robert Millikan (1909), with famous oil drop experiment, discovered basic unit of charge: e = 1. 60 x 10 -19 C u Electrons and protons each have an amount of charge equal to e

Thomson’s Cathode Ray Tube

Millikan’s Experiment

J. J. Thomson Robert Millikan (1868 1953

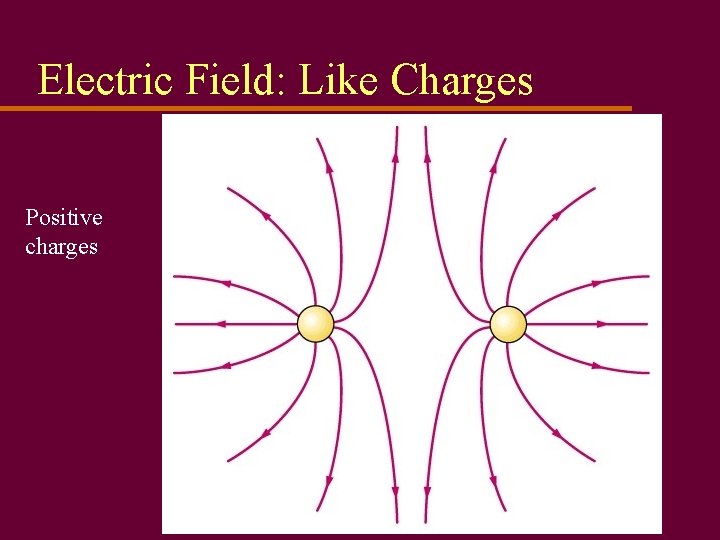
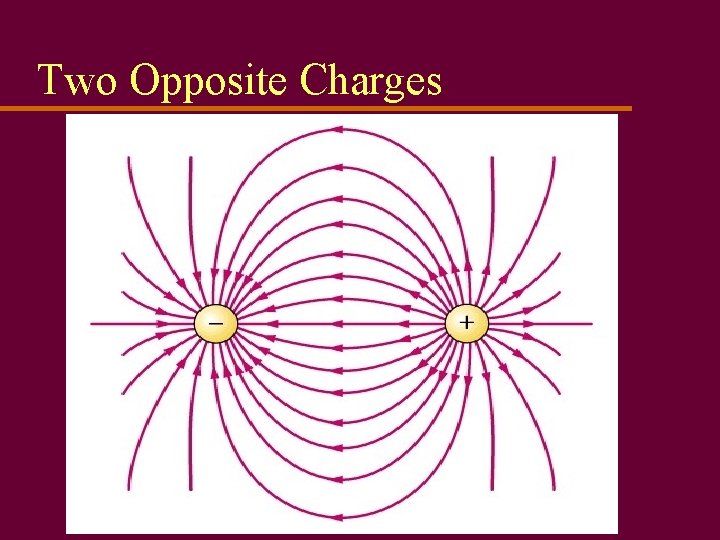
Electrical Forces u Electrical charges exert forces on each other u Law of electrostatics: Like charges repel; opposites attract

Conduction u Conductor: readily transmits electric charge u Insulator: inhibits transfer of charge u Metals are good conductors because of cloud of free electrons surrounding crystal lattice u Electrons tightly bound in insulators u Excess charge placed on insulator stays put in one area; in metals, charge spreads evenly

Charge Transfer u Induction: charged object brought close, but not touching, causes charge separation (polarization) in electroscope (or other object) u Transfer by induction: if connection to ground (infinite charge source or sink) provided while charge is near (so electrons can travel on or off), residual charge of opposite type will remain on electroscope

Charging by Induction: Grounding allows charges to move off sphere leaving opposite residual charge.

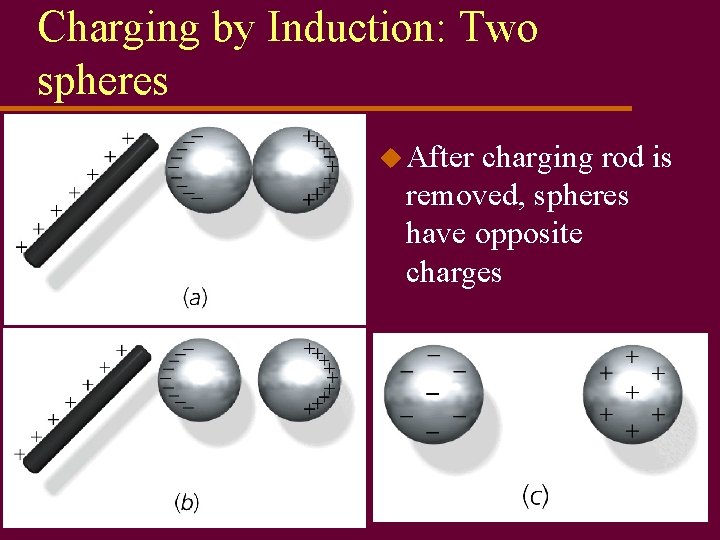
Charging by Induction: Two spheres u After charging rod is removed, spheres have opposite charges

Charge Transfer u Conduction: electrical contact is made u Charging an electroscope by conduction: Charged object brought in contact with electroscope, some of excess charge transferred leaving residual charge of same type on electroscope

Electroscope

Summary u All matter contains huge amounts of + and - charge u Charges can be separated, transferred by contact u Electric charge is conserved and quantized u Like charges repel; opposite charges attract u Conductors have free electrons; insulators inhibit charge flow, electrons bound u Electroscope detects charge state; charged by induction or conduction.

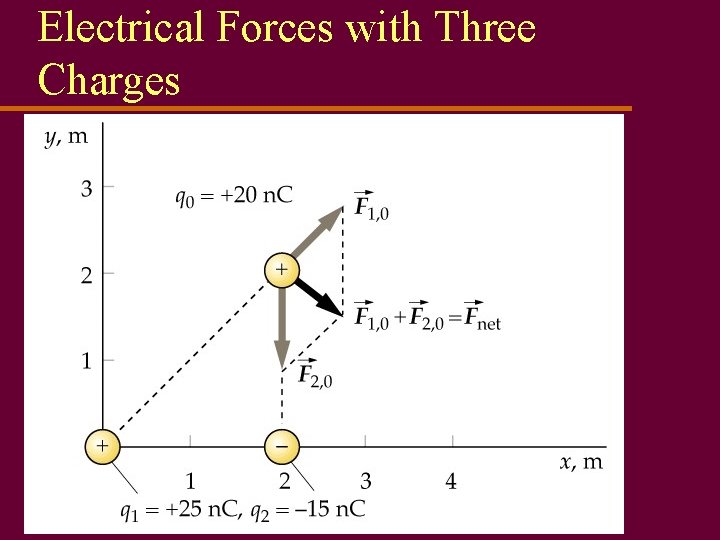
Forces Between Charges u Force between charges obeys law very similar to law of gravitation u For spherical charge distributions, force acts like all charge concentrated at center u Can be attractive (-) or repulsive (+) force u Force directly proportional to product of two charges, inversely prop. to square of distance between charges

Charles Augustin de Coulomb 1736 - 1806

Coulomb’s Law u Realized by many early experimenters, 1785 Coulomb first to quantify with correct constant u Coulomb’s Law: Q = charge in coulombs r = distance between charges k = 8. 99 x 109 Nm 2/C 2 (Coulomb’s constant)

Electrical Forces u Electrical forces are equal and opposite interactions between two charged objects u Like all forces, measured in newtons u If more than two charges are present, forces between each pair of charges are calculated, then vector sum must be found for total force on each charge.

Electrical Forces with Three Charges

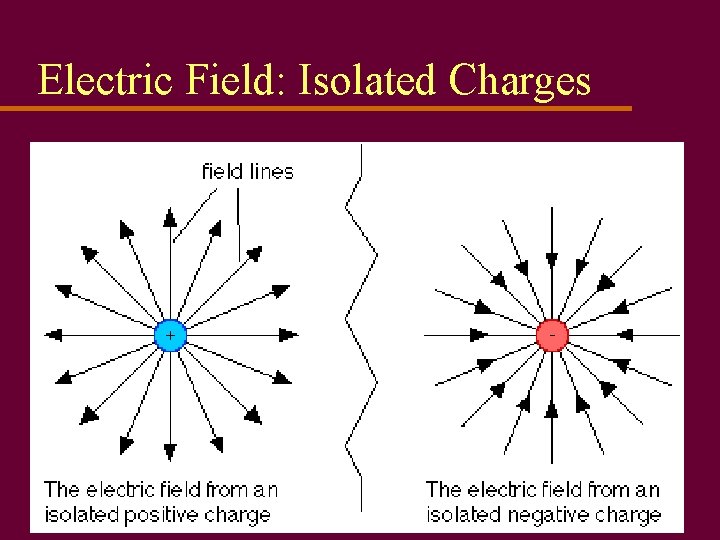
Electric Fields u Proposed by Michael Faraday (1832) to illustrate how forces can act with no contact u Draw lines of force that start at pos. charges and end on neg. charges u Number of lines in area represent strength of field (magnitude)

Electric Fields u Field lines end in arrows like vectors u Arrowheads point towards neg. charge; show direction of force on pos. test charge u Strength of field around a charge, Q, is calculated by using pos. test charge qo (real or imaginary), small enough to be negligible

Electric Field: Isolated Charges

Electric Field: Like Charges Positive charges

Two Opposite Charges

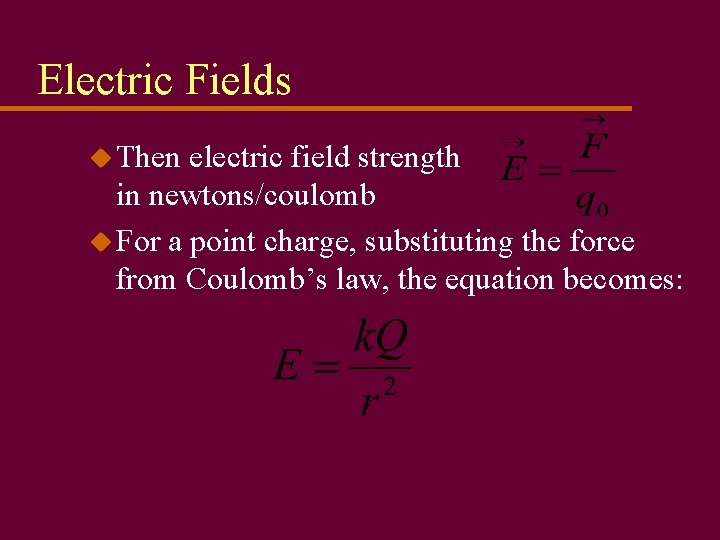
Electric Fields u Then electric field strength in newtons/coulomb u For a point charge, substituting the force from Coulomb’s law, the equation becomes:

Summary u Forces between charges is calculated using Coulomb’s Law, an inverse square law u Electric field is visualized by field lines showing magnitude and direction of force on positive test charge u Field strength expressed in newtons of force per coulomb of charge

Electrostatics Electric Potential

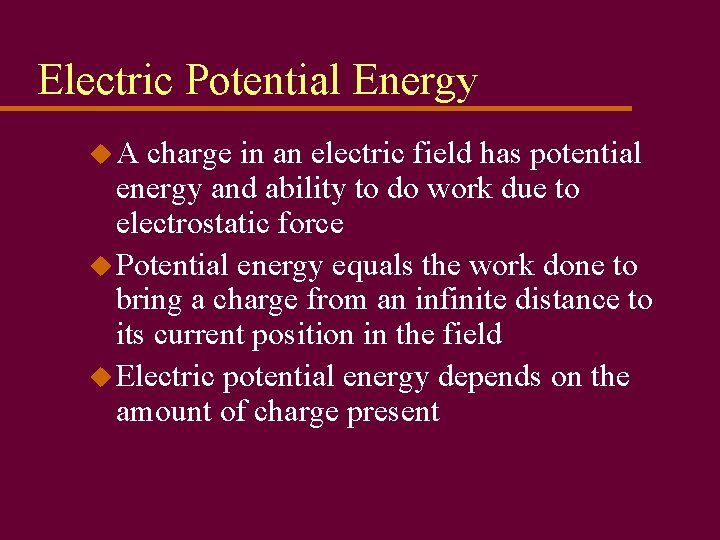
Electric Potential Energy u. A charge in an electric field has potential energy and ability to do work due to electrostatic force u Potential energy equals the work done to bring a charge from an infinite distance to its current position in the field u Electric potential energy depends on the amount of charge present

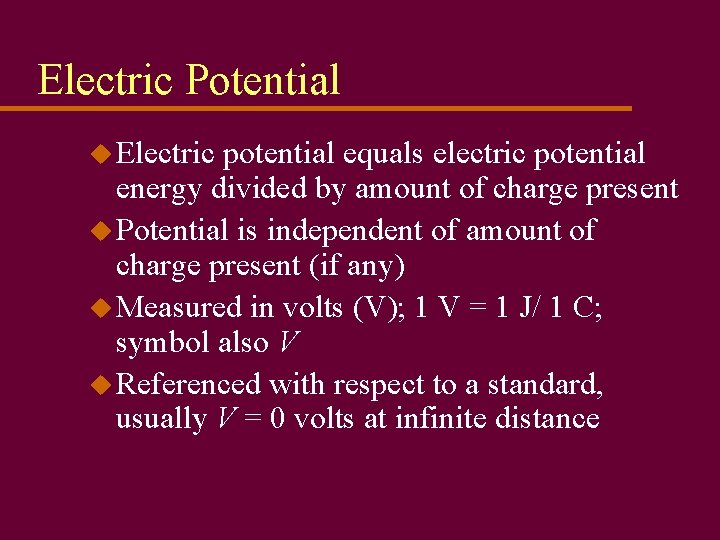
Electric Potential u Electric potential equals electric potential energy divided by amount of charge present u Potential is independent of amount of charge present (if any) u Measured in volts (V); 1 V = 1 J/ 1 C; symbol also V u Referenced with respect to a standard, usually V = 0 volts at infinite distance

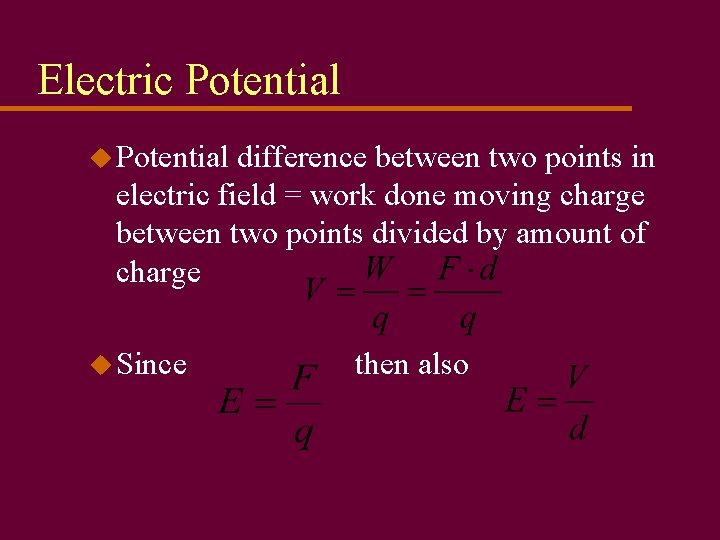
Electric Potential u Potential difference between two points in electric field = work done moving charge between two points divided by amount of charge u Since then also

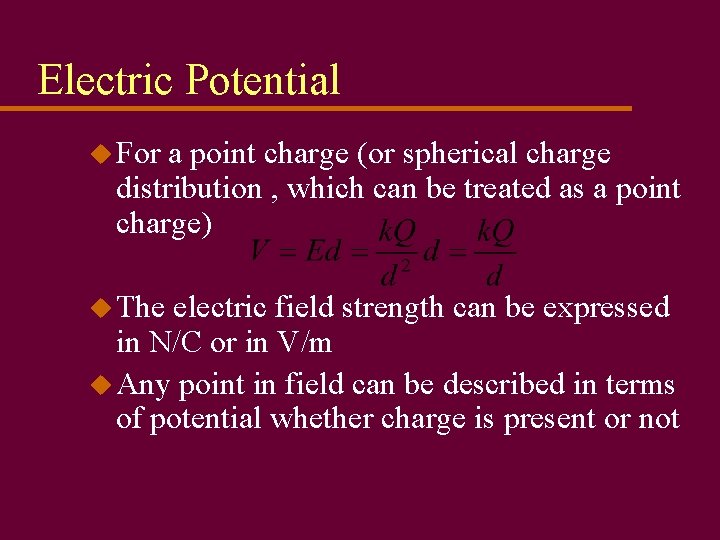
Electric Potential u For a point charge (or spherical charge distribution , which can be treated as a point charge) u The electric field strength can be expressed in N/C or in V/m u Any point in field can be described in terms of potential whether charge is present or not

Grounding u Earth is considered an infinite source or sink for charge - will absorb or give up electrons without changing its overall charge u Earth’s potential considered to be zero u Any object connected to earth is said to be “grounded” (earthed in England) u All building circuitry has wire connected to stake in ground

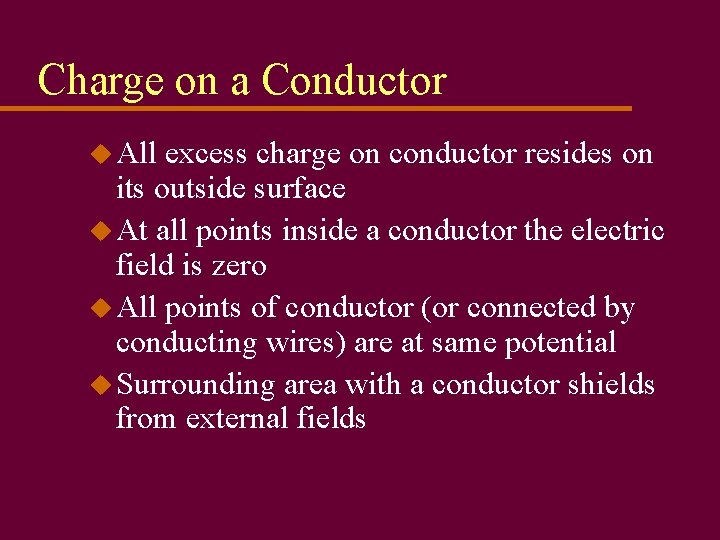
Charge on a Conductor u All excess charge on conductor resides on its outside surface u At all points inside a conductor the electric field is zero u All points of conductor (or connected by conducting wires) are at same potential u Surrounding area with a conductor shields from external fields

Distribution of Charge u If conductor is sphere, charge density will be uniform over surface u For other shapes, charge density varies, more concentrated around points, corners

Distribution of Charge u Spark discharges occur from points: air molecules become ionized into plasma u Lightning is static spark discharge millions of volts potential u Lightning rods create points for spark discharge directing charge to ground - Ben Franklin’s invention

Equipotential Surfaces u Real or imaginary surface surrounding a charge having all points at same potential u In two dimensions, equipotential lines u Equipotential surface always perpendicular to field lines u Point charge has spherical equipotential surfaces

Electrostatics Capacitors and Capacitance

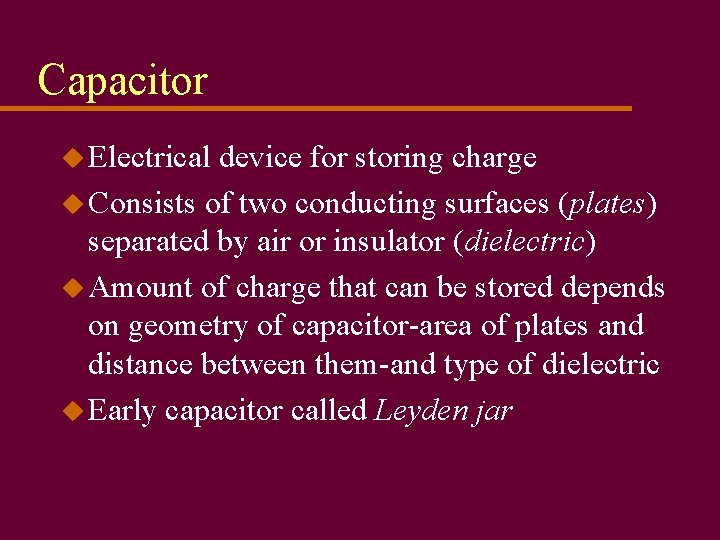
Capacitor u Electrical device for storing charge u Consists of two conducting surfaces (plates) separated by air or insulator (dielectric) u Amount of charge that can be stored depends on geometry of capacitor-area of plates and distance between them-and type of dielectric u Early capacitor called Leyden jar

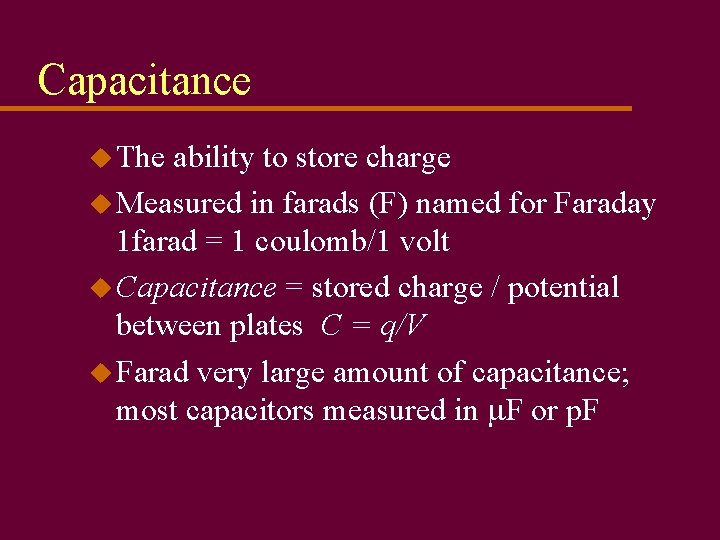
Capacitance u The ability to store charge u Measured in farads (F) named for Faraday 1 farad = 1 coulomb/1 volt u Capacitance = stored charge / potential between plates C = q/V u Farad very large amount of capacitance; most capacitors measured in m. F or p. F

Dielectric u Insulating material between capacitor plates u Increase amount of charge that can be stored by a factor of the material’s dielectric constant, k u k for vacuum = 1, about the same for air u Capacitance increases by factor of k also

Dielectric u For charged cap. not connected to battery, dielectric will reduce potential between plates u Molecules in dielectric become aligned with electric field between plates u This sets up opposing electric field that weakens electric field between plates u Dielectric can be polar or non-polar

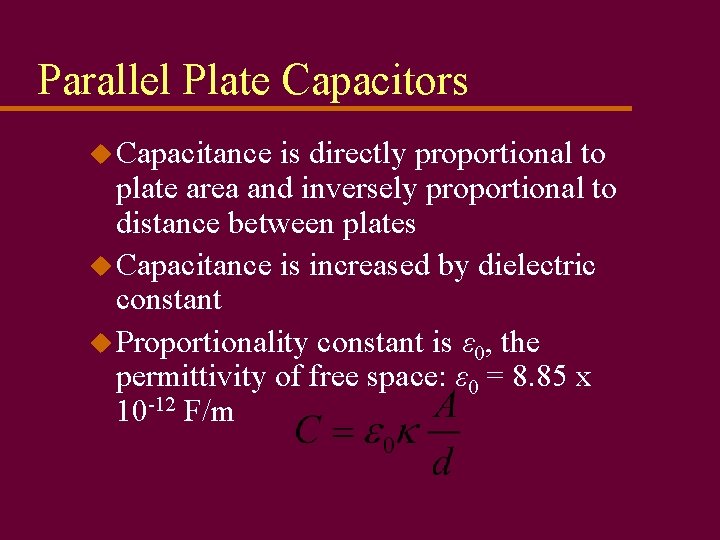
Parallel Plate Capacitors u Capacitance is directly proportional to plate area and inversely proportional to distance between plates u Capacitance is increased by dielectric constant u Proportionality constant is ε 0, the permittivity of free space: ε 0 = 8. 85 x 10 -12 F/m

Stored Energy u Work done moving charge onto plates during charging process is stored as energy in the electric field between the plates u Energy can be used at a later time to do work on charges, moving them as capacitor discharges

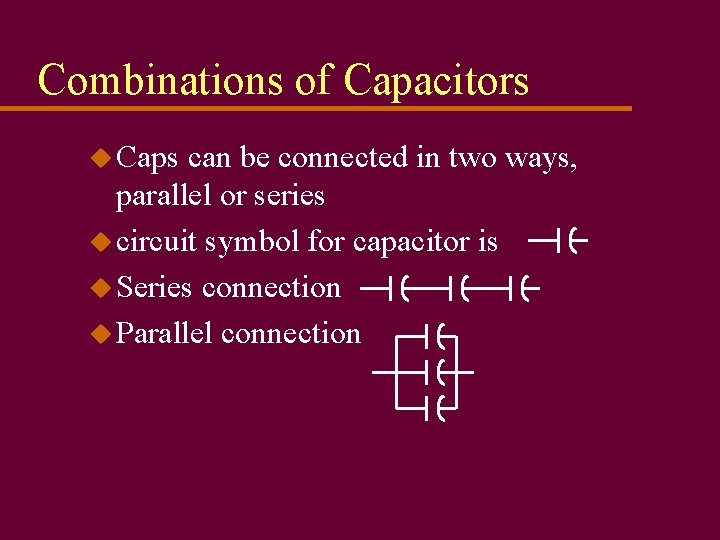
Combinations of Capacitors u Caps can be connected in two ways, parallel or series u circuit symbol for capacitor is u Series connection u Parallel connection

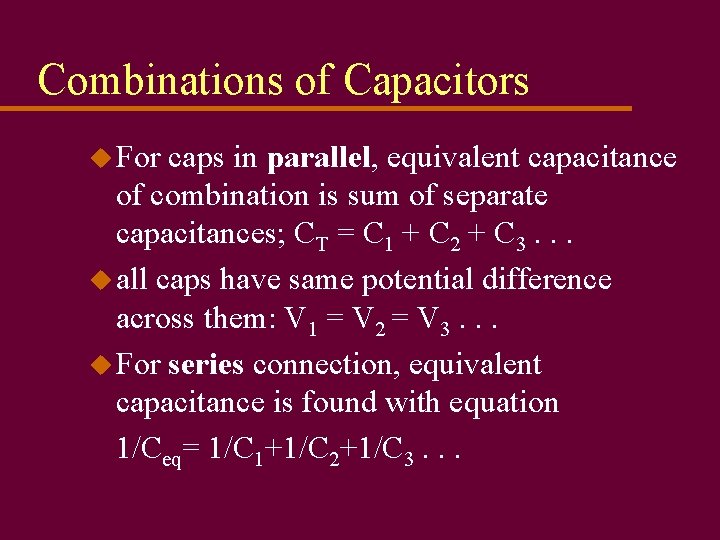
Combinations of Capacitors u For caps in parallel, equivalent capacitance of combination is sum of separate capacitances; CT = C 1 + C 2 + C 3. . . u all caps have same potential difference across them: V 1 = V 2 = V 3. . . u For series connection, equivalent capacitance is found with equation 1/Ceq= 1/C 1+1/C 2+1/C 3. . .

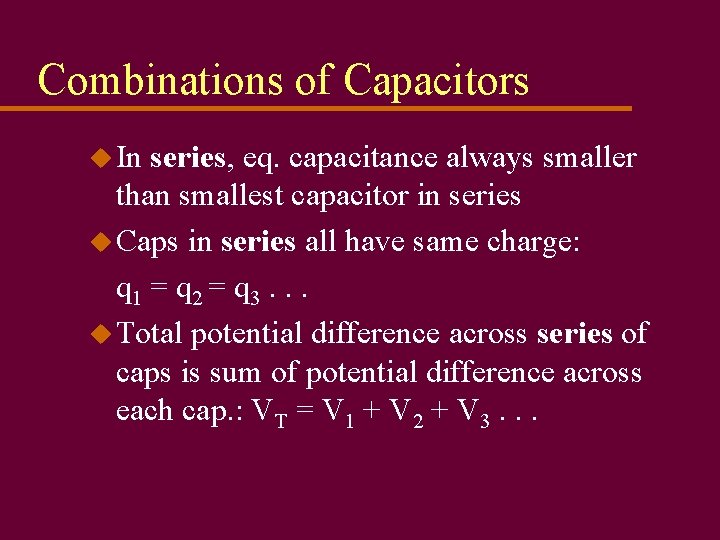
Combinations of Capacitors u In series, eq. capacitance always smaller than smallest capacitor in series u Caps in series all have same charge: q 1 = q 2 = q 3. . . u Total potential difference across series of caps is sum of potential difference across each cap. : VT = V 1 + V 2 + V 3. . .