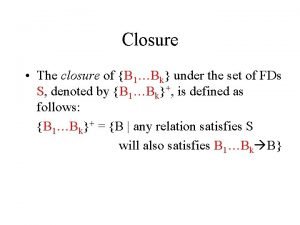
Effects of Varying Acidic Conditions on Ring Closure



![Ø 1 -aza-8 -oxo-4 -oxa-[3. 3. 0]bicyclooctanes These α-hydrogens can be replaced and more Ø 1 -aza-8 -oxo-4 -oxa-[3. 3. 0]bicyclooctanes These α-hydrogens can be replaced and more](https://slidetodoc.com/presentation_image_h2/a937435b62429e79681ace2277cfa03d/image-4.jpg)






- Slides: 20

Effects of Varying Acidic Conditions on Ring Closure of acyliminium Ion Intermediates in the Synthesis of Arylsubstituted bicyclic lactams: Finding Optimal Conditions for Increasing Yields. Teresa Phan Dr. Buonora California State University, Long Beach

q Introduction q Bicyclic Lactams q Making Bicyclic Lactam q Experiment: Varying p. H on Ring Closure q Anticipated Results q Concluding Remarks

Ø Libraries of dihydropyridazinones and hydroxamic acids as potential pharmaceutical uses and advancement in organic synthesis Ø Ex: inodilators and phosphodiesterase inhibitors Ø Find conditions to optimize yields of each reaction to increase efficiency Ø Bicyclic Lactams are important starting materials
![Ø 1 aza8 oxo4 oxa3 3 0bicyclooctanes These αhydrogens can be replaced and more Ø 1 -aza-8 -oxo-4 -oxa-[3. 3. 0]bicyclooctanes These α-hydrogens can be replaced and more](https://slidetodoc.com/presentation_image_h2/a937435b62429e79681ace2277cfa03d/image-4.jpg)
Ø 1 -aza-8 -oxo-4 -oxa-[3. 3. 0]bicyclooctanes These α-hydrogens can be replaced and more functional groups added Ø Vehicles for the construction of natural and unnatural products containing quaternary carbon centers

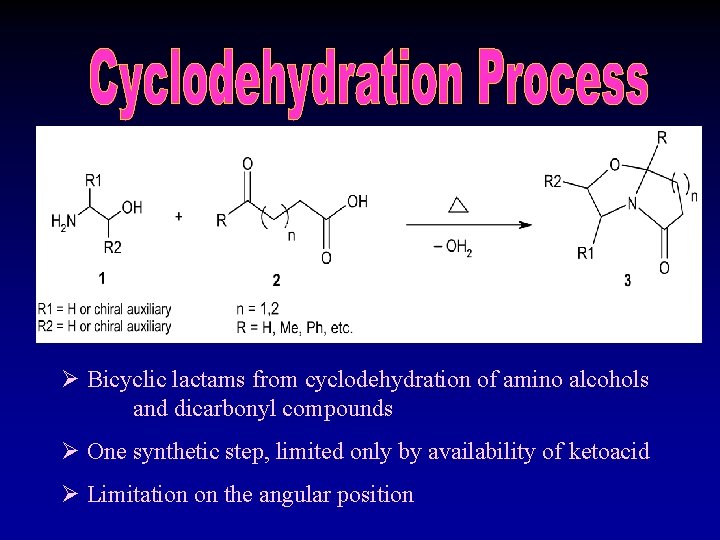
Ø Bicyclic lactams from cyclodehydration of amino alcohols and dicarbonyl compounds Ø One synthetic step, limited only by availability of ketoacid Ø Limitation on the angular position

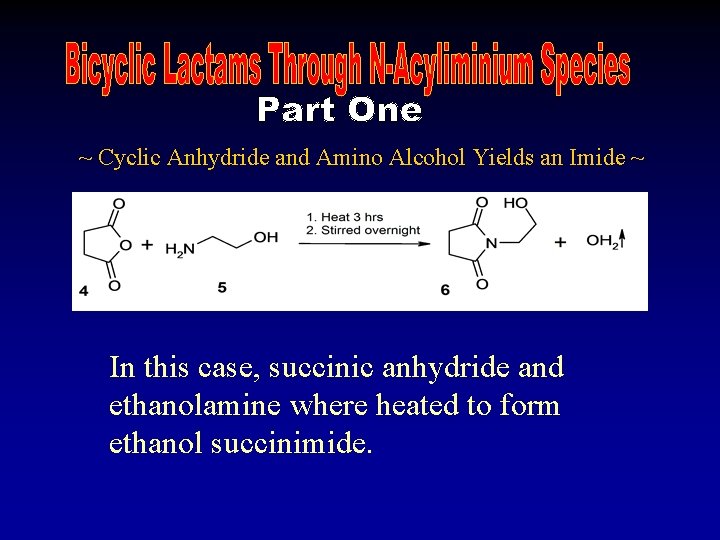
~ Cyclic Anhydride and Amino Alcohol Yields an Imide ~ In this case, succinic anhydride and ethanolamine where heated to form ethanol succinimide.

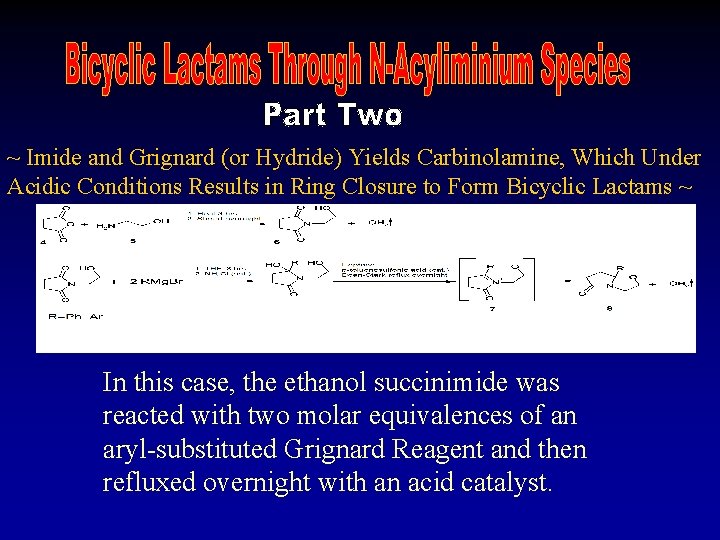
~ Imide and Grignard (or Hydride) Yields Carbinolamine, Which Under Acidic Conditions Results in Ring Closure to Form Bicyclic Lactams ~ In this case, the ethanol succinimide was reacted with two molar equivalences of an aryl-substituted Grignard Reagent and then refluxed overnight with an acid catalyst.

(2 -step synthesis involving N-acyliminium species) • Grignard reagents can add a greater variety of R groups at the angular position • Better yields than the respective cyclodehydration route • Previous studies indicate that reagent stoichiometry and solution p. H during closure may directly affect yields

v Uses Grignard Reagents: RMg. Br v Grignard Reagents are Very Reactive Nucleophiles v Concentration Deteriorate With Time, Moisture and Air v True Concentrations Found by Direct Titration Using sec-Butanol in Xylene and 1, 10 -Phenanthroline as Indicator v Grignard Reagents Should be Titrated Once a Month

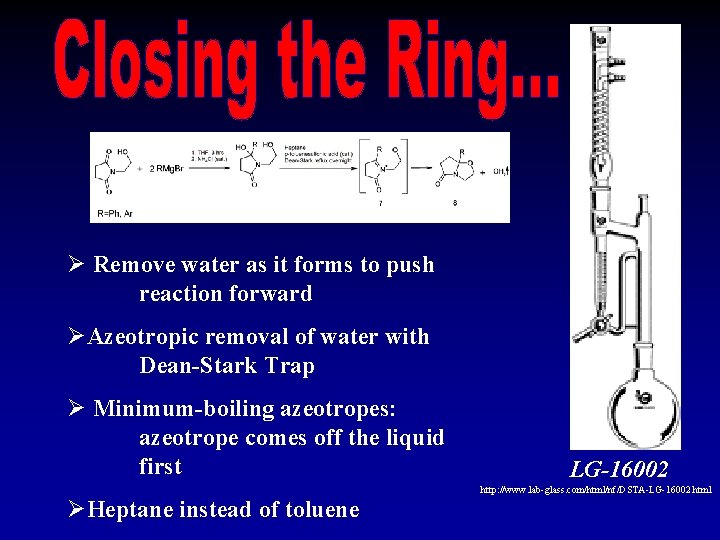
Ø Remove water as it forms to push reaction forward ØAzeotropic removal of water with Dean-Stark Trap Ø Minimum-boiling azeotropes: azeotrope comes off the liquid first ØHeptane instead of toluene LG-16002 http: //www. lab-glass. com/html/nf/DSTA-LG-16002. html

Ø Run TLC, visualize with vanillin solution Ø Push through silica gel plug Ø Radial Chromatography (Chromatotron) Ø Collect Fractions in Tubes Ø Run TLC to Combine Tubes Ø Evaporate off Solvent Via Rotary Evaporator Ø Analyze by NMR Ø Find Percent Yield by Weight of Purified Product

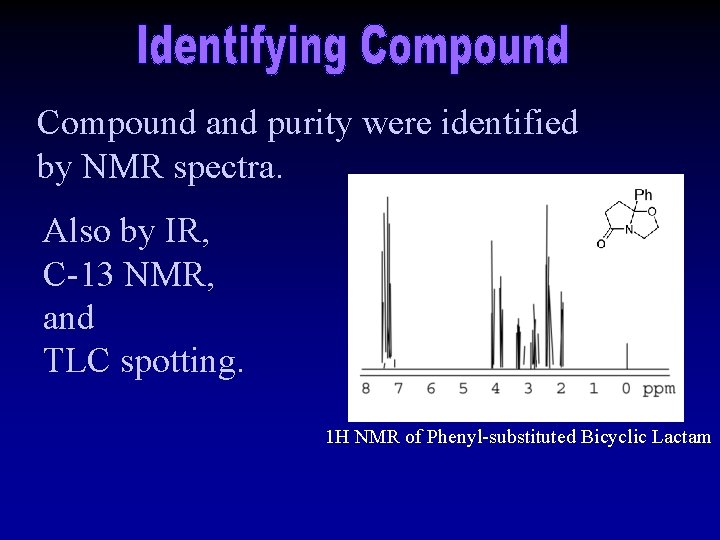
Compound and purity were identified by NMR spectra. Also by IR, C-13 NMR, and TLC spotting. 1 H NMR of Phenyl-substituted Bicyclic Lactam

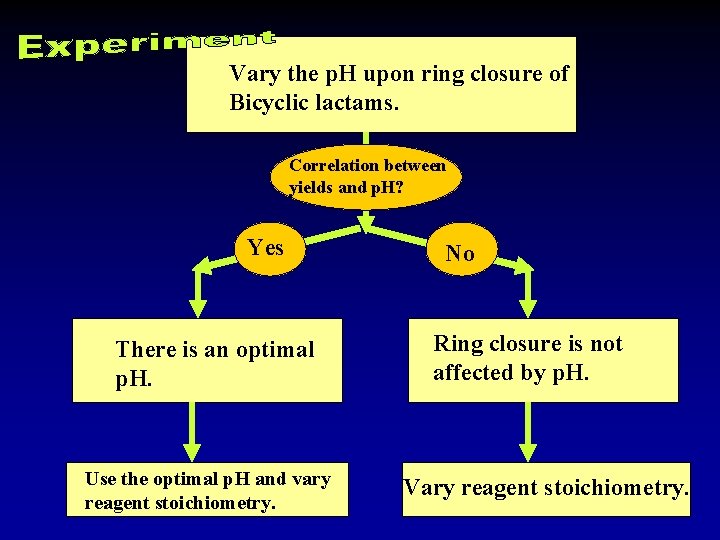
Vary the p. H upon ring closure of Bicyclic lactams. Correlation between yields and p. H? Yes There is an optimal p. H. Use the optimal p. H and vary reagent stoichiometry. No Ring closure is not affected by p. H. Vary reagent stoichiometry.

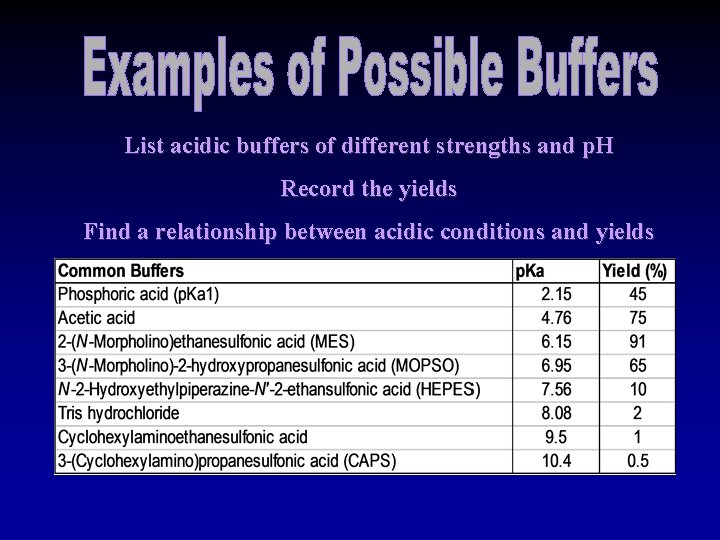
List acidic buffers of different strengths and p. H Record the yields Find a relationship between acidic conditions and yields

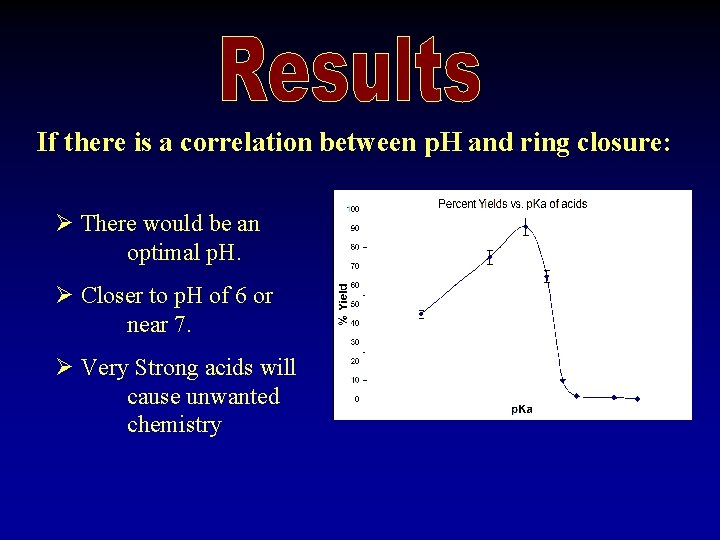
If there is a correlation between p. H and ring closure: Ø There would be an optimal p. H. Ø Closer to p. H of 6 or near 7. Ø Very Strong acids will cause unwanted chemistry

Ø We will find out if p. H affects the yields by looking for peaked yields at a specific p. H Ø It may be that the p. H does not matter as much as the type of acid used Ø If we can’t get yields to increase to >90%, then we have to look at reaction preferences as well as reagent stoichiometry

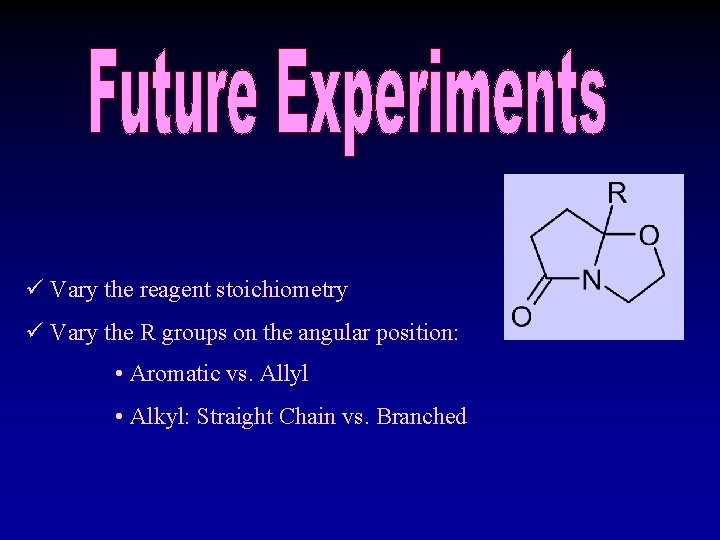
ü Vary the reagent stoichiometry ü Vary the R groups on the angular position: • Aromatic vs. Allyl • Alkyl: Straight Chain vs. Branched

Industrial Size Distillation Column Ø Future Applications: • Dihydropyridazinone and Hydroxamic Acid derivatives • Medicine • Safer Insecticides • Natural and Unnatural Products with Quaternary Carbon Centers http: //www. aut. ee. ethz. ch/~disti/

ØCalifornia State University, Long Beach (Department of Chemistry and Biochemistry and the Department of Biological Sciences) ØDr. Paul Buonora ØDr. Mason and Dr. Archie ØThe Howard Hughes Medical Institute

 Internal ring
Internal ring Token ring and resilient packet ring
Token ring and resilient packet ring What is the brown ring in the brown ring test
What is the brown ring in the brown ring test Ding dong ding dong christmas bells are ringing
Ding dong ding dong christmas bells are ringing Strong no in c
Strong no in c Ring christmas bells ring them loud
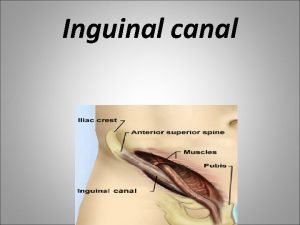
Ring christmas bells ring them loud Hesselbach's triangle borders
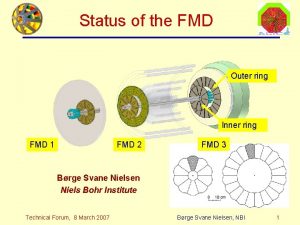
Hesselbach's triangle borders Inner ring and outer ring
Inner ring and outer ring Particle on a ring
Particle on a ring What is varied sentence structure
What is varied sentence structure Cobol perform varying decrement
Cobol perform varying decrement Openers for sentences
Openers for sentences Cobol search varying
Cobol search varying Maxwell's equations faraday's law
Maxwell's equations faraday's law Work done by a varying force
Work done by a varying force Adverb sentence starters
Adverb sentence starters Varying sentence beginnings
Varying sentence beginnings Exit sentence
Exit sentence Equation of continuity for time varying fields
Equation of continuity for time varying fields Varying sentence structures
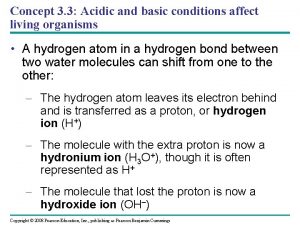
Varying sentence structures Acidic ocean
Acidic ocean