Chapter 7 Ring closure and ring opening 7

- Slides: 22

Chapter 7 Ring closure (and ring opening) 7. 1 Intramolecular cyclization by electrophilenucleophile 7. 2 Cycloadditon 7. 3 Electrocyclic ring closure 7. 4 Ring opening 1

7. 1 Intramolecular cyclization by electrophilenucleophile 7. 1. 1 Introduction (alkylation, acylation and condensation etc. ) 2

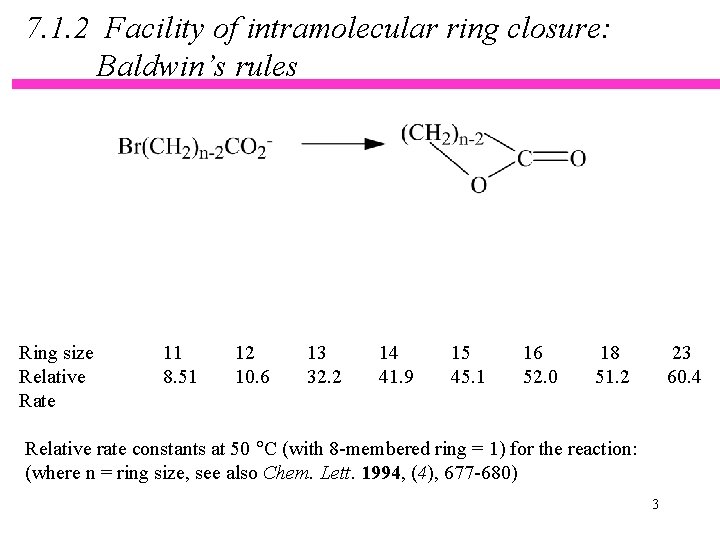
7. 1. 2 Facility of intramolecular ring closure: Baldwin’s rules Ring size Relative Rate 11 8. 51 12 10. 6 13 32. 2 14 41. 9 15 45. 1 16 52. 0 18 51. 2 23 60. 4 Relative rate constants at 50 C (with 8 -membered ring = 1) for the reaction: (where n = ring size, see also Chem. Lett. 1994, (4), 677 -680) 3

4

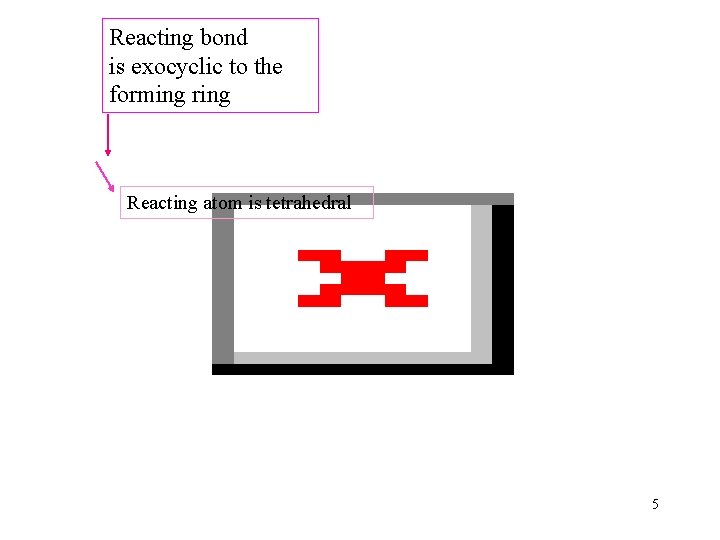
Reacting bond is exocyclic to the forming ring Reacting atom is tetrahedral 5

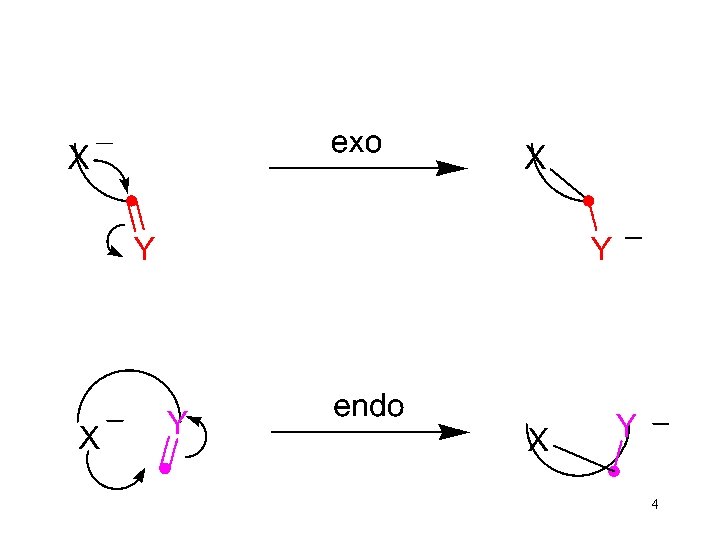
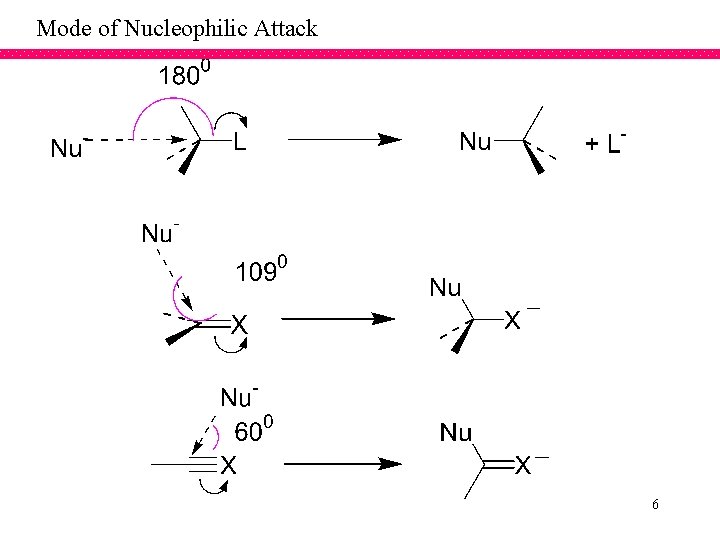
Mode of Nucleophilic Attack 6

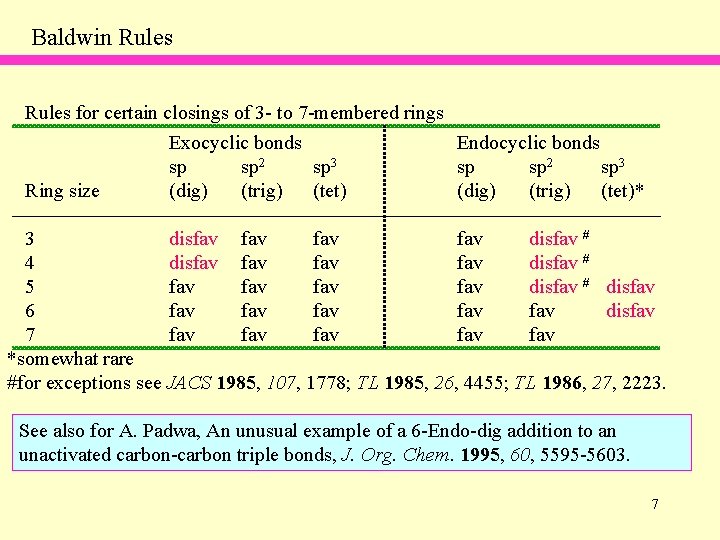
Baldwin Rules for certain closings of 3 - to 7 -membered rings Ring size Exocyclic bonds sp sp 2 sp 3 (dig) (trig) (tet) Endocyclic bonds sp sp 2 sp 3 (dig) (trig) (tet)* 3 disfav fav disfav # 4 disfav fav disfav # 5 fav fav disfav # disfav 6 fav fav fav disfav 7 fav fav fav *somewhat rare #for exceptions see JACS 1985, 107, 1778; TL 1985, 26, 4455; TL 1986, 27, 2223. See also for A. Padwa, An unusual example of a 6 -Endo-dig addition to an unactivated carbon-carbon triple bonds, J. Org. Chem. 1995, 60, 5595 -5603. 7

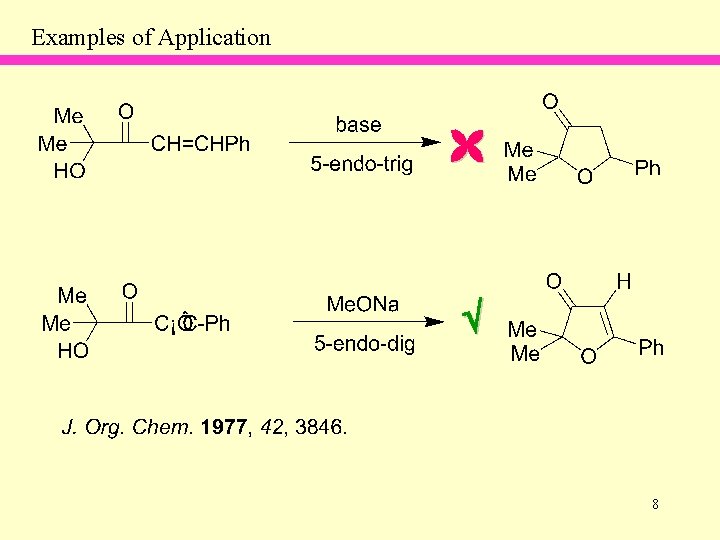
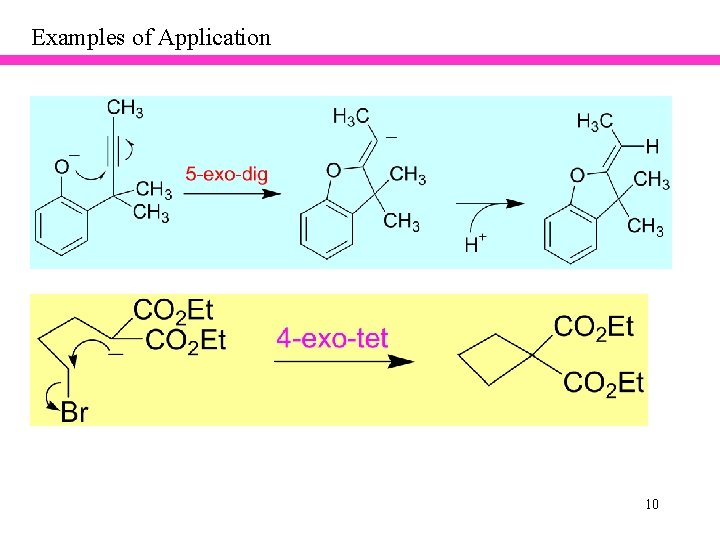
Examples of Application 8

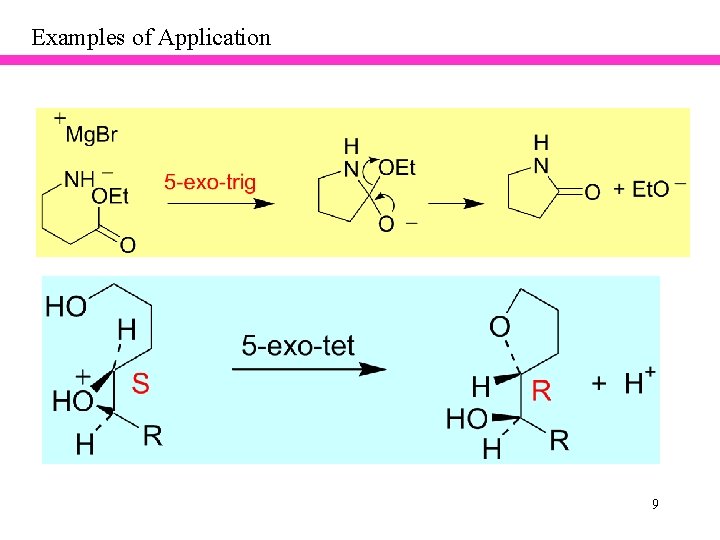
Examples of Application 9

Examples of Application 10

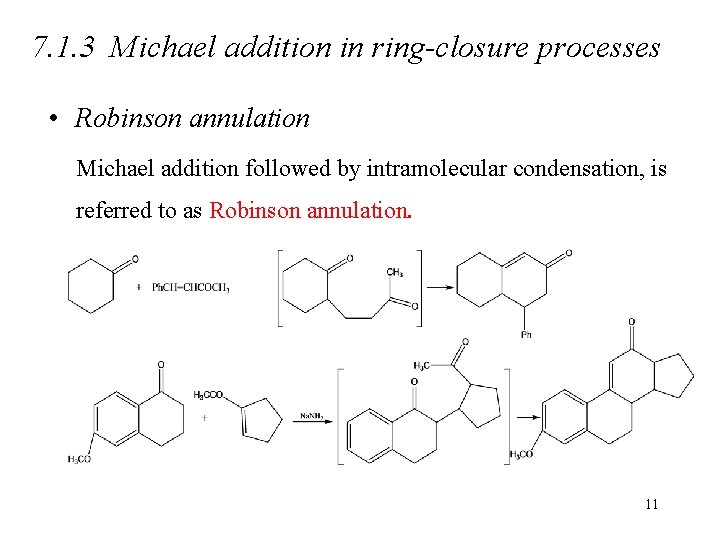
7. 1. 3 Michael addition in ring-closure processes • Robinson annulation Michael addition followed by intramolecular condensation, is referred to as Robinson annulation. 11

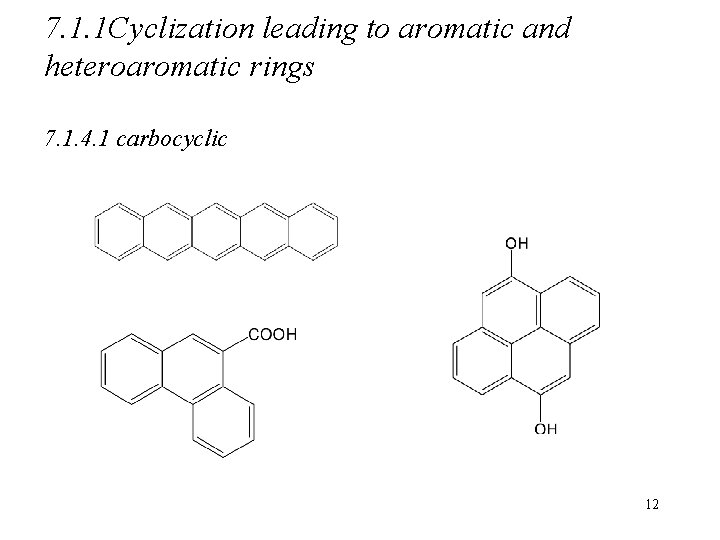
7. 1. 1 Cyclization leading to aromatic and heteroaromatic rings 7. 1. 4. 1 carbocyclic 12

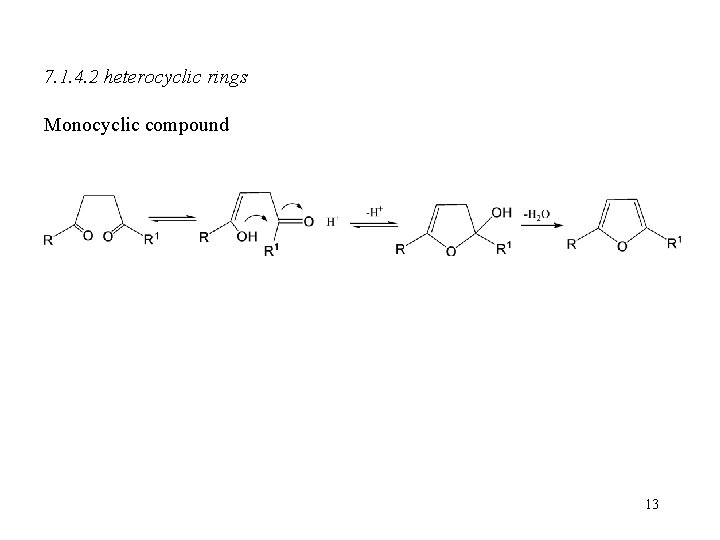
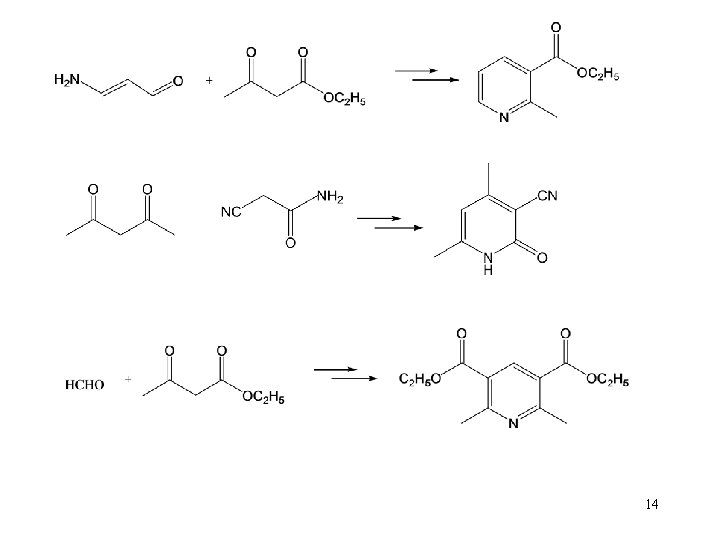
7. 1. 4. 2 heterocyclic rings Monocyclic compound 13

14

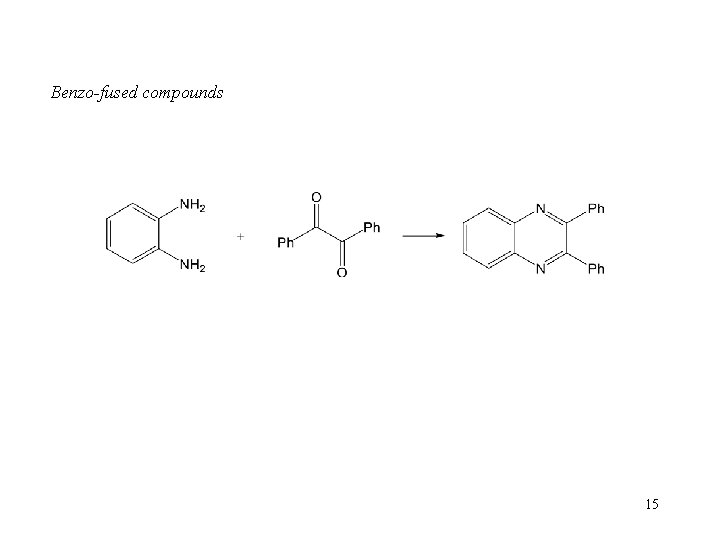
Benzo-fused compounds 15

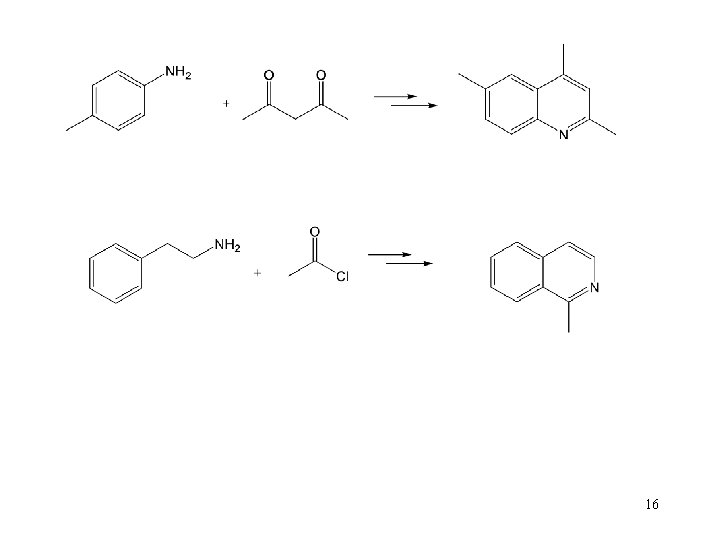
16

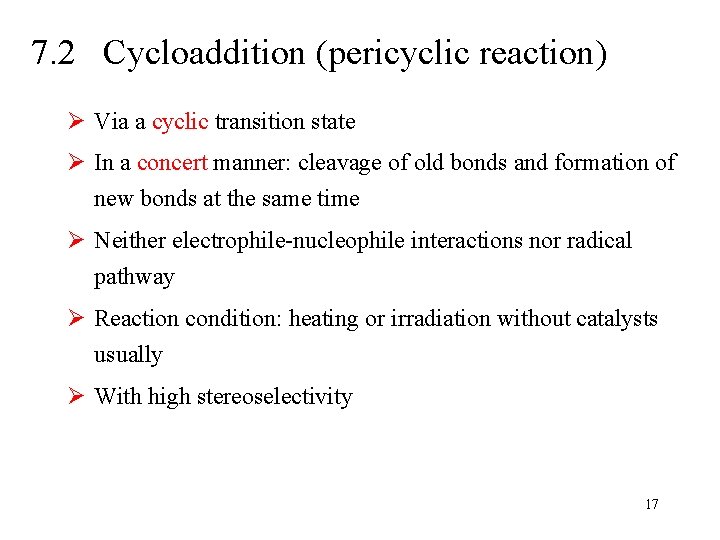
7. 2 Cycloaddition (pericyclic reaction) Ø Via a cyclic transition state Ø In a concert manner: cleavage of old bonds and formation of new bonds at the same time Ø Neither electrophile-nucleophile interactions nor radical pathway Ø Reaction condition: heating or irradiation without catalysts usually Ø With high stereoselectivity 17

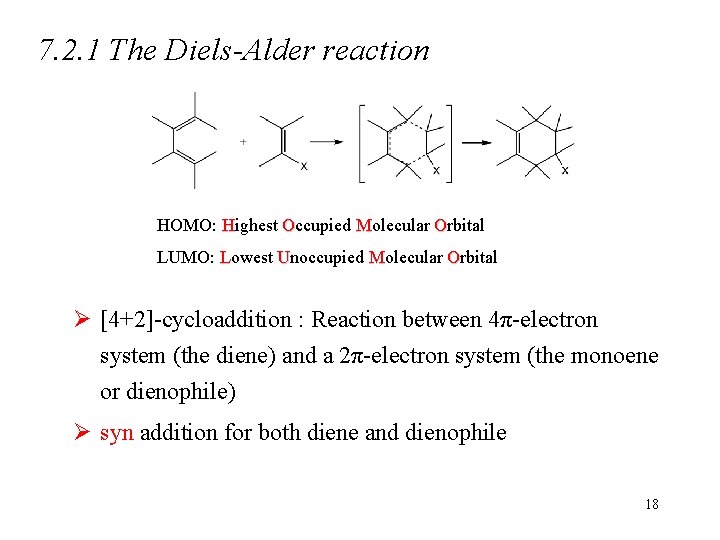
7. 2. 1 The Diels-Alder reaction HOMO: Highest Occupied Molecular Orbital LUMO: Lowest Unoccupied Molecular Orbital Ø [4+2]-cycloaddition : Reaction between 4π-electron system (the diene) and a 2π-electron system (the monoene or dienophile) Ø syn addition for both diene and dienophile 18

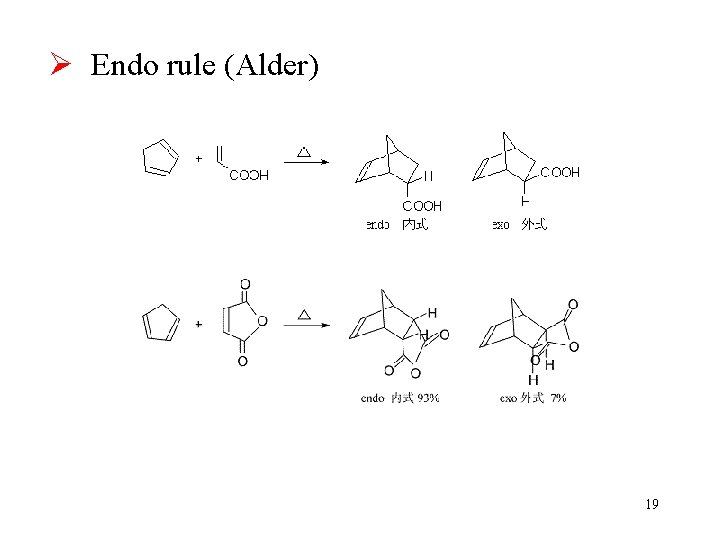
Ø Endo rule (Alder) 19

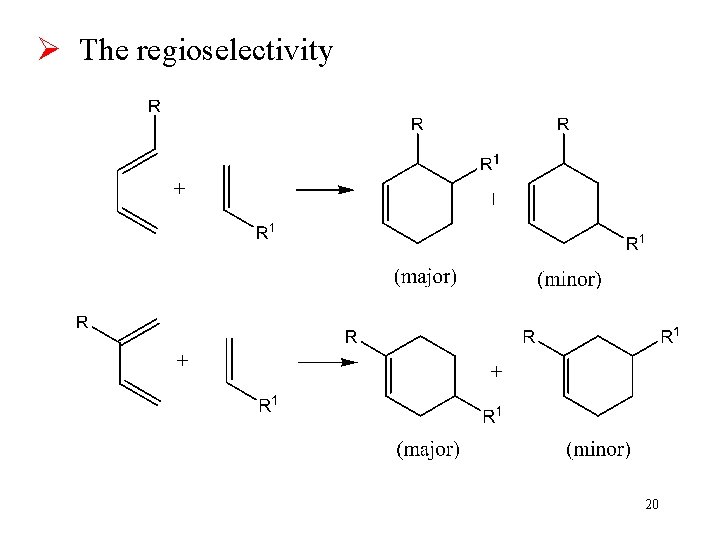
Ø The regioselectivity 20

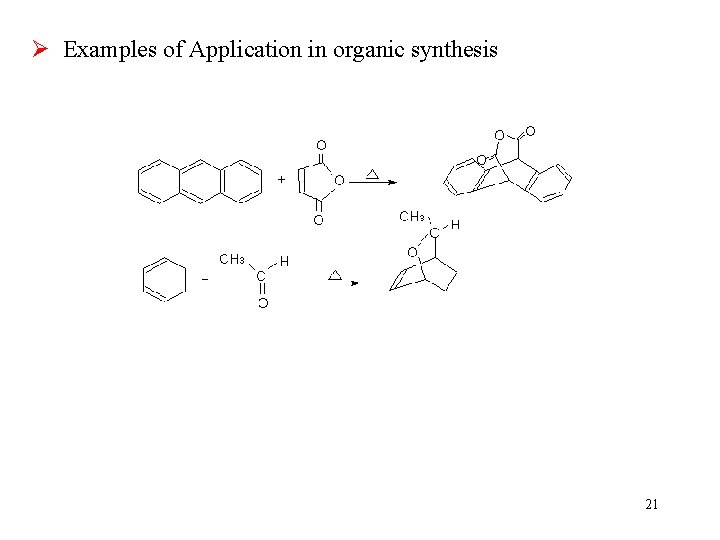
Ø Examples of Application in organic synthesis 21

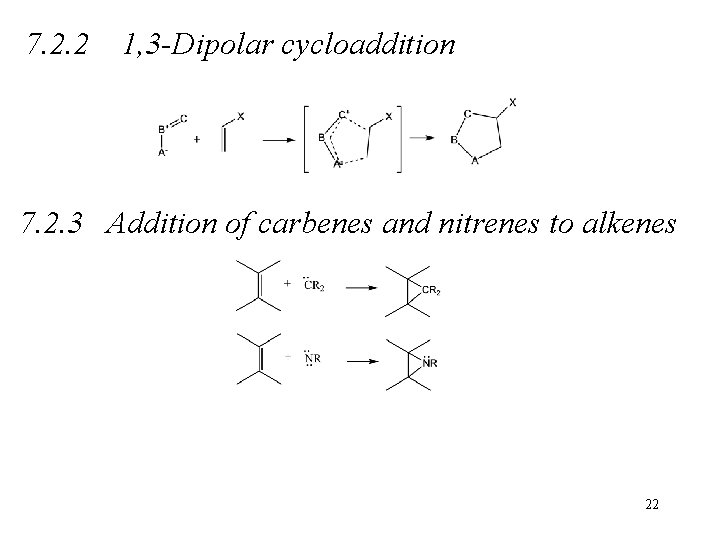
7. 2. 2 1, 3 -Dipolar cycloaddition 7. 2. 3 Addition of carbenes and nitrenes to alkenes 22