ECLAIR Phase 2 A Safety and PK Study



- Slides: 17

ECLAIR: Phase 2 A Safety and PK Study of Cabotegravir LA in HIV-Uninfected Men Martin Markowitz, 1 Ian Frank, 2 Robert M. Grant, 3 Kenneth H. Mayer, 4 David A. Margolis, 5 Krischan J. Hudson, 5 Britt S. Stancil, 6 Susan L. Ford, 6 Alex R. Rinehart, 5 William R. Spreen 5 1 The Aaron Diamond AIDS Research Center, an affiliate of the Rockefeller University, New York, NY; School of Medicine, University of Pennsylvania, Philadelphia, PA; 3 Institute of Virology & Immunology, Gladstone Institutes, San Francisco, CA; Department of Medicine, University of California, San Francisco, CA; 4 The Fenway Institute, Fenway Health, Boston, MA; Beth Israel Deaconess Medical Center, Boston, MA; Harvard Medical School, Boston, MA; 5 Vii. V Healthcare, Research Triangle Park, NC; 6 PAREXEL International (formerly employed by Glaxo. Smith. Kline), Research Triangle Park, NC 2 Perelman 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

Disclosures • Dr. Martin Markowitz has received research grants from GSK/Vii. V and Gilead Sciences awarded to his institution • Dr. Markowitz has served as a consultant to Merck • Dr. Markowitz is a speaker on behalf of Gilead Sciences Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

Introduction • Daily oral Pr. EP has proven effective in randomized clinical trials in MSM, discordant couples, and IDUs, but less so in other populations • Efficacy strongly related to adherence • LA injections may overcome barriers to adherence • CAB is a potent INSTI that has been formulated in an LA suspension CAB, cabotegravir; IDU, intravenous drug user; INSTI, integrase strand transfer inhibitor; LA, long-acting; MSM, men who have sex with men; Pr. EP, pre-exposure prophylaxis. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

Objectives Primary • To evaluate the safety and tolerability of IM CAB LA injections through Week 41 Secondary • To evaluate the pharmacokinetics of CAB LA injection through Week 41 • To evaluate the safety and tolerability of oral CAB • To assess the acceptability of CAB LA injections IM, intramuscular. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

Study Population Key Inclusion Criteria • Healthy men 18 to 65 years of age • At low risk of acquiring HIV, defined as having at least one casual sex partner in the past 24 months Key Exclusion Criteria • At high risk for HIV infection • Recent use of ART (eg, for PEP or Pr. EP) in the past 30 days or 5 half-lives • Current or chronic history of liver disease ART, antiretroviral therapy; PEP, post-exposure prophylaxis. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

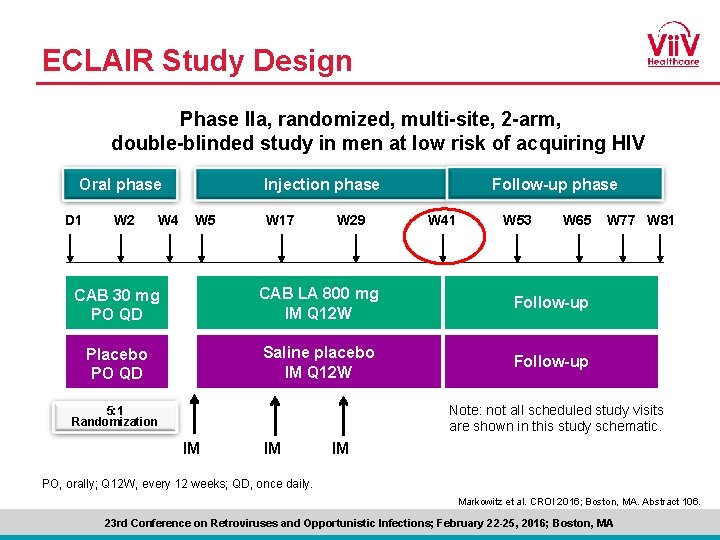
ECLAIR Study Design Phase IIa, randomized, multi-site, 2 -arm, double-blinded study in men at low risk of acquiring HIV Oral phase D 1 W 2 W 4 Follow-up phase Injection phase W 5 W 17 W 29 W 41 W 53 W 65 CAB 30 mg PO QD CAB LA 800 mg IM Q 12 W Follow-up Placebo PO QD Saline placebo IM Q 12 W Follow-up W 77 W 81 Note: not all scheduled study visits are shown in this study schematic. 5: 1 Randomization IM IM IM PO, orally; Q 12 W, every 12 weeks; QD, once daily. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

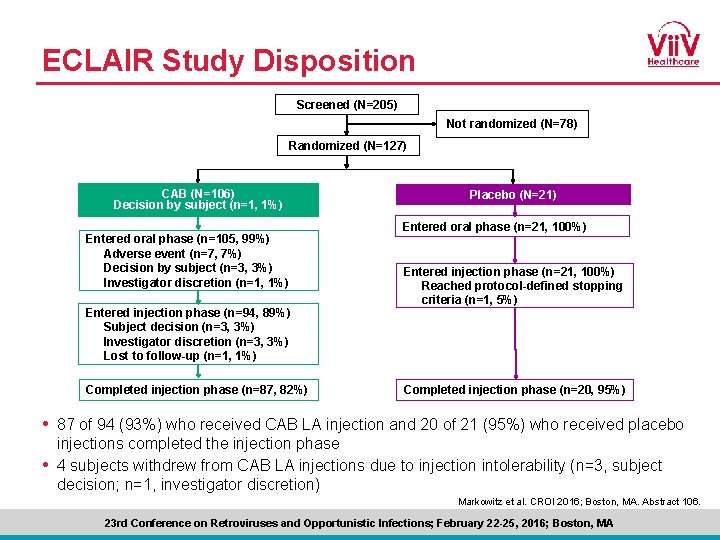
ECLAIR Study Disposition Screened (N=205) Not randomized (N=78) Randomized (N=127) CAB (N=106) Decision by subject (n=1, 1%) Entered oral phase (n=105, 99%) Adverse event (n=7, 7%) Decision by subject (n=3, 3%) Investigator discretion (n=1, 1%) Entered injection phase (n=94, 89%) Subject decision (n=3, 3%) Investigator discretion (n=3, 3%) Lost to follow-up (n=1, 1%) Completed injection phase (n=87, 82%) Placebo (N=21) Entered oral phase (n=21, 100%) Entered injection phase (n=21, 100%) Reached protocol-defined stopping criteria (n=1, 5%) Completed injection phase (n=20, 95%) • 87 of 94 (93%) who received CAB LA injection and 20 of 21 (95%) who received placebo injections completed the injection phase • 4 subjects withdrew from CAB LA injections due to injection intolerability (n=3, subject decision; n=1, investigator discretion) Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

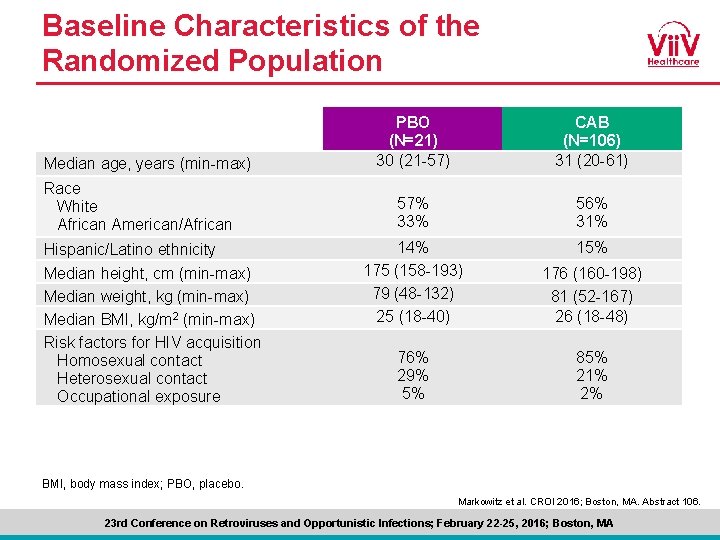
Baseline Characteristics of the Randomized Population Median age, years (min-max) Race White African American/African Hispanic/Latino ethnicity Median height, cm (min-max) Median weight, kg (min-max) Median BMI, kg/m 2 (min-max) Risk factors for HIV acquisition Homosexual contact Heterosexual contact Occupational exposure PBO (N=21) 30 (21 -57) CAB (N=106) 31 (20 -61) 57% 33% 56% 31% 14% 175 (158 -193) 79 (48 -132) 25 (18 -40) 15% 176 (160 -198) 81 (52 -167) 26 (18 -48) 76% 29% 5% 85% 21% 2% BMI, body mass index; PBO, placebo. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

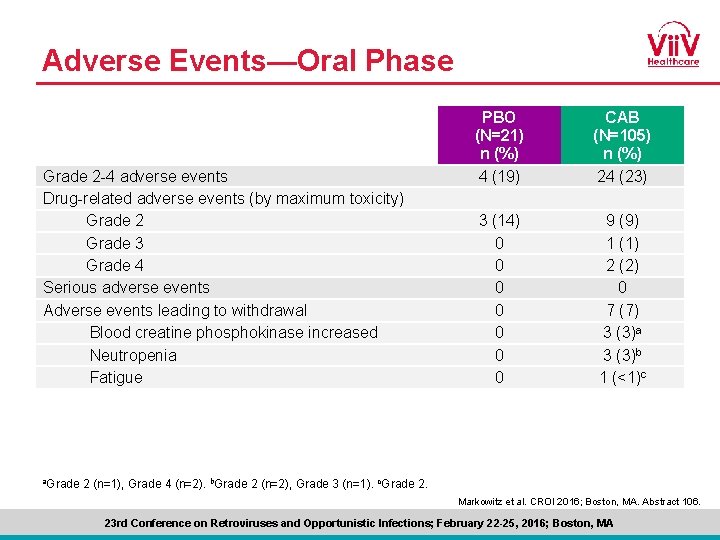
Adverse Events—Oral Phase Grade 2 -4 adverse events Drug-related adverse events (by maximum toxicity) Grade 2 Grade 3 Grade 4 Serious adverse events Adverse events leading to withdrawal Blood creatine phosphokinase increased Neutropenia Fatigue a. Grade PBO (N=21) n (%) 4 (19) CAB (N=105) n (%) 24 (23) 3 (14) 0 0 0 0 9 (9) 1 (1) 2 (2) 0 7 (7) 3 (3)a 3 (3)b 1 (<1)c 2 (n=1), Grade 4 (n=2). b. Grade 2 (n=2), Grade 3 (n=1). c. Grade 2. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

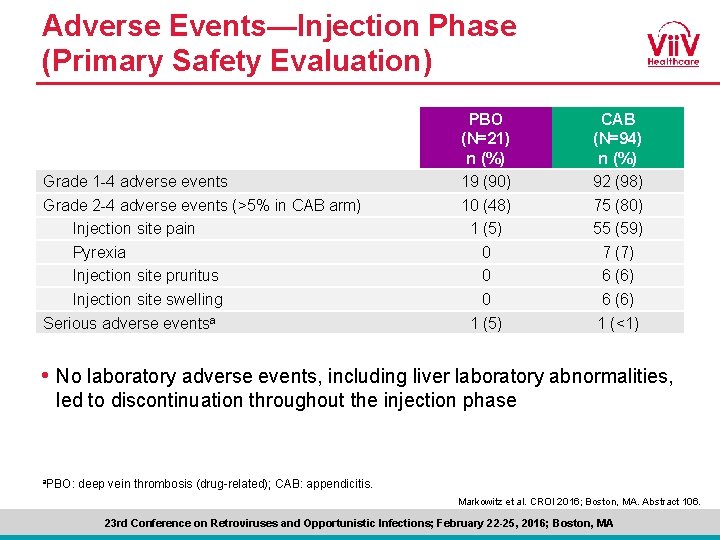
Adverse Events—Injection Phase (Primary Safety Evaluation) Grade 1 -4 adverse events Grade 2 -4 adverse events (>5% in CAB arm) Injection site pain Pyrexia Injection site pruritus Injection site swelling Serious adverse eventsa PBO (N=21) n (%) 19 (90) 10 (48) 1 (5) 0 0 0 1 (5) CAB (N=94) n (%) 92 (98) 75 (80) 55 (59) 7 (7) 6 (6) 1 (<1) • No laboratory adverse events, including liver laboratory abnormalities, led to discontinuation throughout the injection phase a. PBO: deep vein thrombosis (drug-related); CAB: appendicitis. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

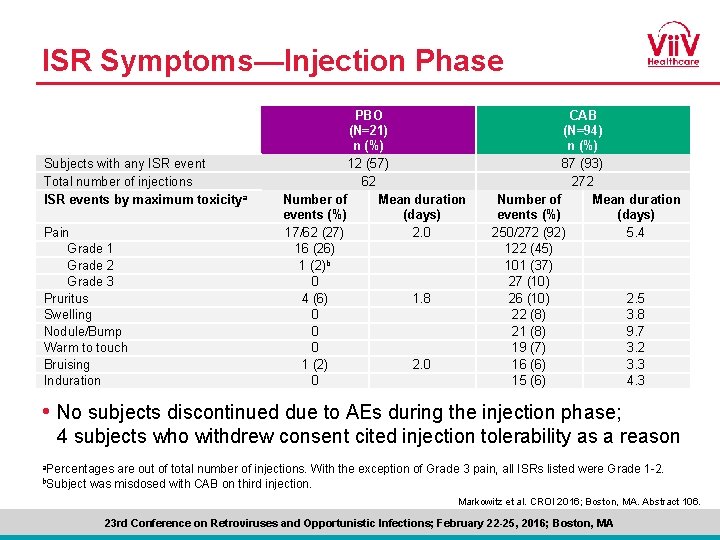
ISR Symptoms—Injection Phase Subjects with any ISR event Total number of injections ISR events by maximum toxicity a Pain Grade 1 Grade 2 Grade 3 Pruritus Swelling Nodule/Bump Warm to touch Bruising Induration PBO (N=21) n (%) 12 (57) 62 Number of Mean duration events (%) (days) 17/62 (27) 2. 0 16 (26) 1 (2)b 0 4 (6) 1. 8 0 0 0 1 (2) 2. 0 0 CAB (N=94) n (%) 87 (93) 272 Number of Mean duration events (%) (days) 250/272 (92) 5. 4 122 (45) 101 (37) 27 (10) 26 (10) 2. 5 22 (8) 3. 8 21 (8) 9. 7 19 (7) 3. 2 16 (6) 3. 3 15 (6) 4. 3 • No subjects discontinued due to AEs during the injection phase; 4 subjects who withdrew consent cited injection tolerability as a reason a. Percentages b. Subject are out of total number of injections. With the exception of Grade 3 pain, all ISRs listed were Grade 1 -2. was misdosed with CAB on third injection. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

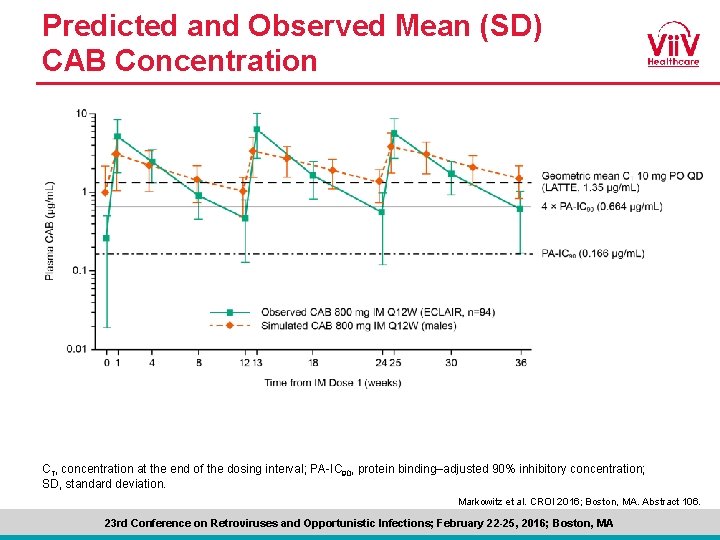
Predicted and Observed Mean (SD) CAB Concentration CT, concentration at the end of the dosing interval; PA-IC 90, protein binding–adjusted 90% inhibitory concentration; SD, standard deviation. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

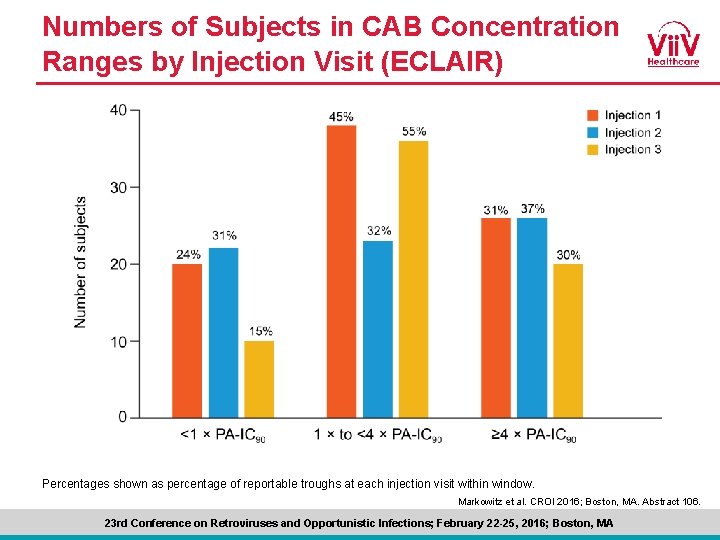
Numbers of Subjects in CAB Concentration Ranges by Injection Visit (ECLAIR) Percentages shown as percentage of reportable troughs at each injection visit within window. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

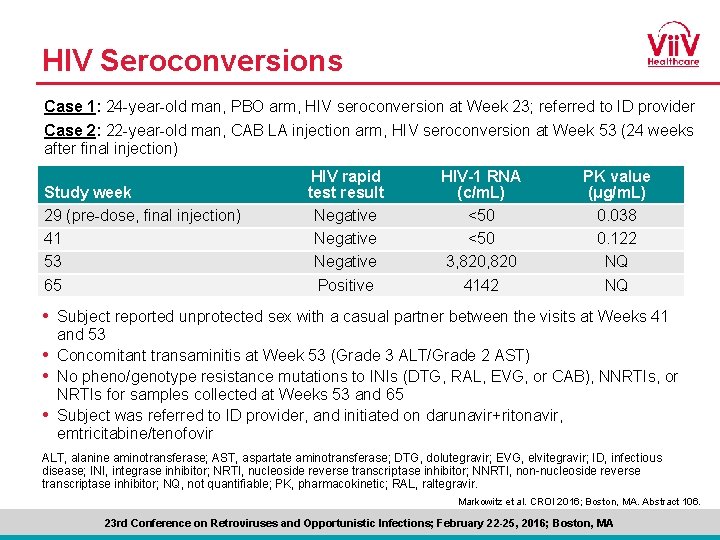
HIV Seroconversions Case 1: 24 -year-old man, PBO arm, HIV seroconversion at Week 23; referred to ID provider Case 2: 22 -year-old man, CAB LA injection arm, HIV seroconversion at Week 53 (24 weeks after final injection) Study week 29 (pre-dose, final injection) 41 53 65 HIV rapid test result Negative Positive HIV-1 RNA (c/m. L) <50 3, 820 4142 PK value (μg/m. L) 0. 038 0. 122 NQ NQ • Subject reported unprotected sex with a casual partner between the visits at Weeks 41 and 53 • Concomitant transaminitis at Week 53 (Grade 3 ALT/Grade 2 AST) • No pheno/genotype resistance mutations to INIs (DTG, RAL, EVG, or CAB), NNRTIs, or NRTIs for samples collected at Weeks 53 and 65 • Subject was referred to ID provider, and initiated on darunavir+ritonavir, emtricitabine/tenofovir ALT, alanine aminotransferase; AST, aspartate aminotransferase; DTG, dolutegravir; EVG, elvitegravir; ID, infectious disease; INI, integrase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NQ, not quantifiable; PK, pharmacokinetic; RAL, raltegravir. Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

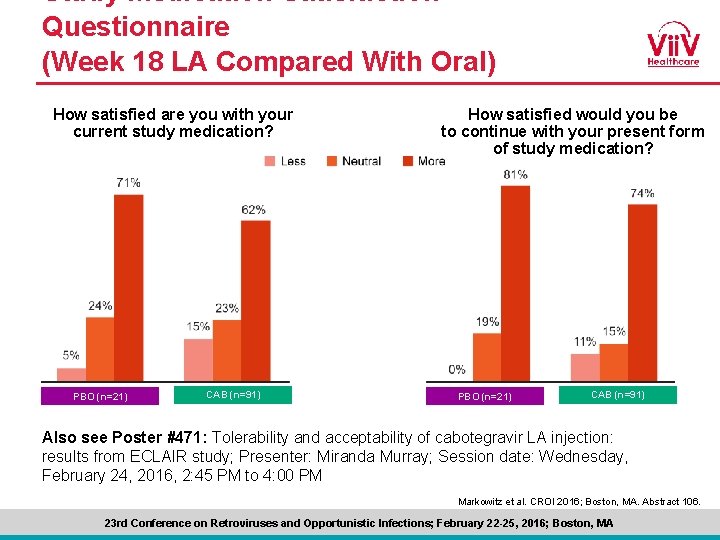
Study Medication Satisfaction Questionnaire (Week 18 LA Compared With Oral) How satisfied are you with your current study medication? PBO (n=21) CAB (n=91) How satisfied would you be to continue with your present form of study medication? PBO (n=21) CAB (n=91) Also see Poster #471: Tolerability and acceptability of cabotegravir LA injection: results from ECLAIR study; Presenter: Miranda Murray; Session date: Wednesday, February 24, 2016, 2: 45 PM to 4: 00 PM Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

ECLAIR Conclusions • Both CAB oral and LA were well tolerated, permitting continued development of CAB for Pr. EP • The absorption rate following CAB LA injection was faster than predicted by early PK population models, leading to higher peak and lower trough exposures • 15% to 31% of trough concentrations were <PA-IC 90, whereas 30% to 37% were ≥ 4 × PA-IC 90 across injection visits, below initial predictions • Given observed trough levels, an 8 -week dosing interval is currently under evaluation • Participant satisfaction with IM CAB LA injections was high, including a preference for injections Q 12 W compared with oral CAB once-daily tablets Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA

Acknowledgments • We thank everyone who has contributed to the success of this study, including – All study participants and their families – The ECLAIR clinical investigators and their staff Investigator Martin Markowitz (PI) Rick Elion/Deborah Goldstein Chester Fisher Ian Frank Joel Gallant Robert Grant Beryl Koblin/Hong Van Tieu Ken Mayer Magdalena Sobieszczyk Winkler Weinberg Clinical site ADARC Whitman Walker Health Research of Hampton Roads, Inc University of Pennsylvania Southwest Care Center Gladstone, UCSF New York Blood Center Harvard/Fenway Institute Columbia University Medical Center Kaiser Permanente – The GSK and Vii. V Healthcare study team – PPD – Quest, Covance, and Monogram Biosciences • This study was funded by Vii. V Healthcare City, State New York, NY Washington, DC Newport News, VA Philadelphia, PA Santa Fe, NM San Francisco, CA New York, NY Boston, MA New York, NY Atlanta, GA Markowitz et al. CROI 2016; Boston, MA. Abstract 106. 23 rd Conference on Retroviruses and Opportunistic Infections; February 22 -25, 2016; Boston, MA