Dynam X Technology Clinical Update A Novel Device

- Slides: 25

Dynam. X Technology & Clinical Update A Novel Device Adapting to Patient Physiology Alex Abizaid, MD Ph. D*, Antonio Colombo, MD, Stefan Verheye, MD In. Cor, Sao Paulo, Brazil

Disclosure Statement of Financial Interest Affiliation/Financial Relationship Company Proctor for TAVR Boston Scientific, Edwards Research grants / Consulting Elixir, Meril Life Science

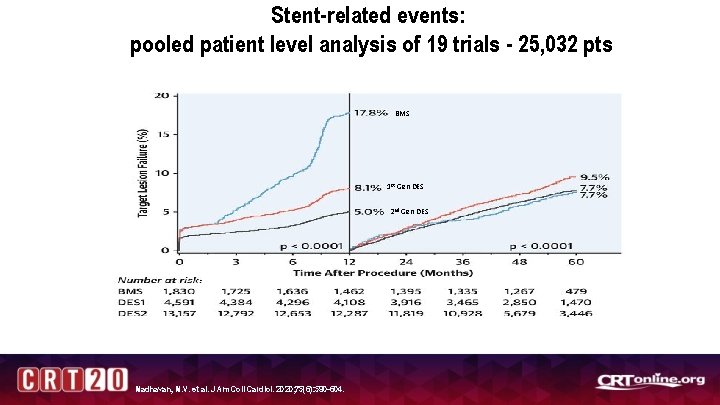
Stent-related events: pooled patient level analysis of 19 trials - 25, 032 pts BMS 1 st Gen DES 2 nd Gen DES Madhavan, M. V. et al. J Am Coll Cardiol. 2020; 75(6): 590 -604.

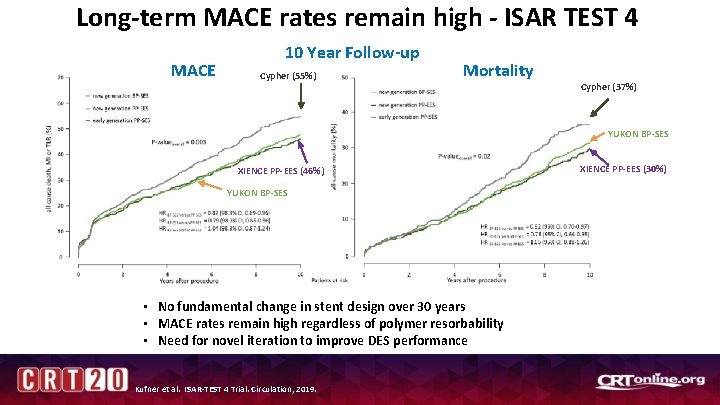
Long-term MACE rates remain high - ISAR TEST 4 MACE 10 Year Follow-up Cypher (55%) Mortality Cypher (37%) YUKON BP-SES XIENCE PP-EES (46%) YUKON BP-SES • No fundamental change in stent design over 30 years • MACE rates remain high regardless of polymer resorbability • Need for novel iteration to improve DES performance Kufner et al. ISAR-TEST 4 Trial. Circulation, 2019. XIENCE PP-EES (30%)

Dynam. X Bioadaptor First fundamental innovation in implant design intended to meet and exceed 2 nd Gen DES performance • Meets acute performance of DES • Exceeds DES performance with unique uncaging of the vessel • Positive adaptive vessel remodeling • Restoration of pulsatility and physiologic cyclic strain • Restoration of vessel angulation

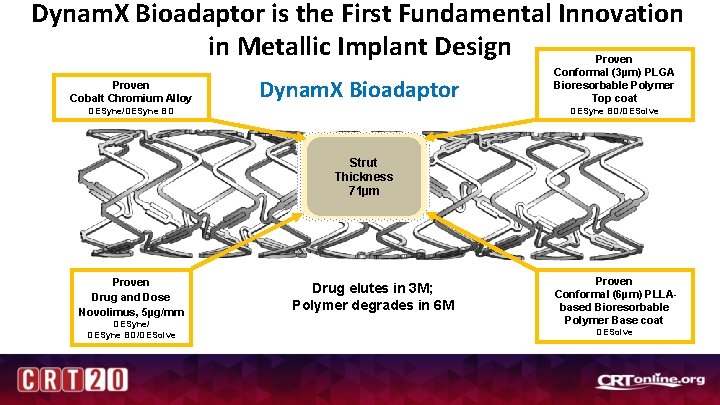
Dynam. X Bioadaptor is the First Fundamental Innovation in Metallic Implant Design Proven Cobalt Chromium Alloy Dynam. X Bioadaptor Proven Conformal (3µm) PLGA Bioresorbable Polymer Top coat DESyne BD/DESolve DESyne/DESyne BD Strut Thickness 71µm Proven Drug and Dose Novolimus, 5µg/mm DESyne/ DESyne BD/DESolve Drug elutes in 3 M; Polymer degrades in 6 M Proven Conformal (6µm) PLLAbased Bioresorbable Polymer Base coat DESolve

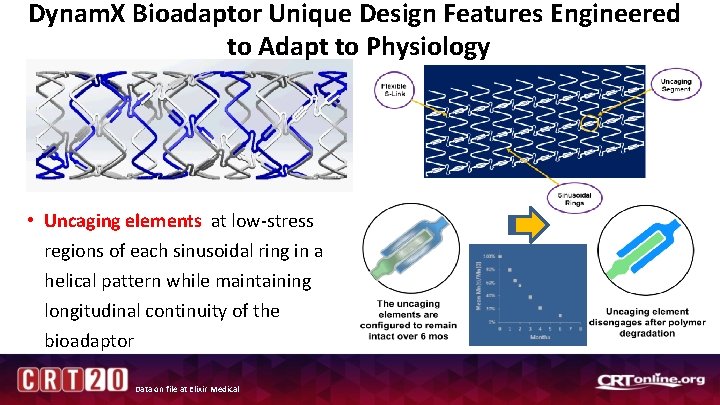
Dynam. X Bioadaptor Unique Design Features Engineered to Adapt to Physiology • Uncaging elements at low-stress regions of each sinusoidal ring in a helical pattern while maintaining longitudinal continuity of the bioadaptor Data on file at Elixir Medical

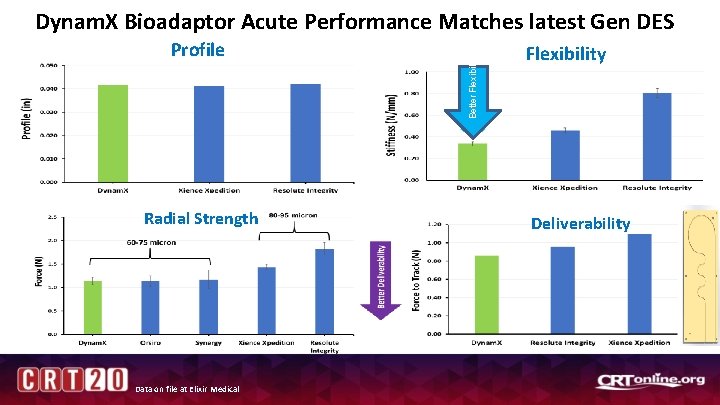
Profile Radial Strength Data on file at Elixir Medical Better Flexibility Dynam. X Bioadaptor Acute Performance Matches latest Gen DES Flexibility Deliverability

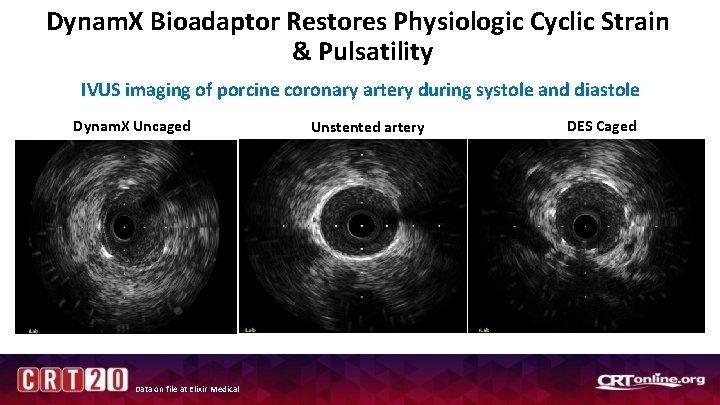
Dynam. X Bioadaptor Restores Physiologic Cyclic Strain & Pulsatility IVUS imaging of porcine coronary artery during systole and diastole Dynam. X Uncaged Data on file at Elixir Medical Unstented artery DES Caged

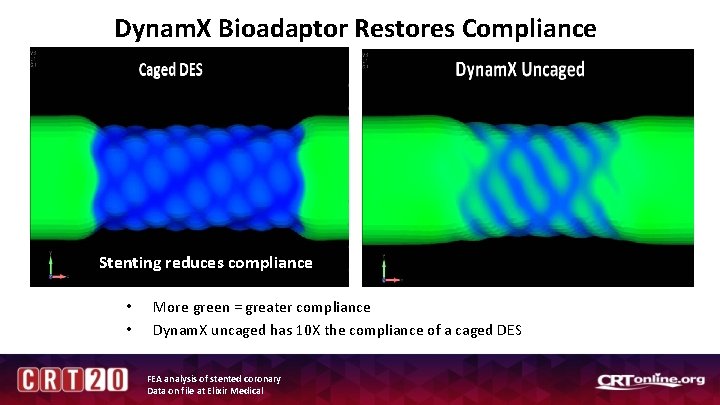
Dynam. X Bioadaptor Restores Compliance Stenting reduces compliance • • More green = greater compliance Dynam. X uncaged has 10 X the compliance of a caged DES FEA analysis of stented coronary Data on file at Elixir Medical

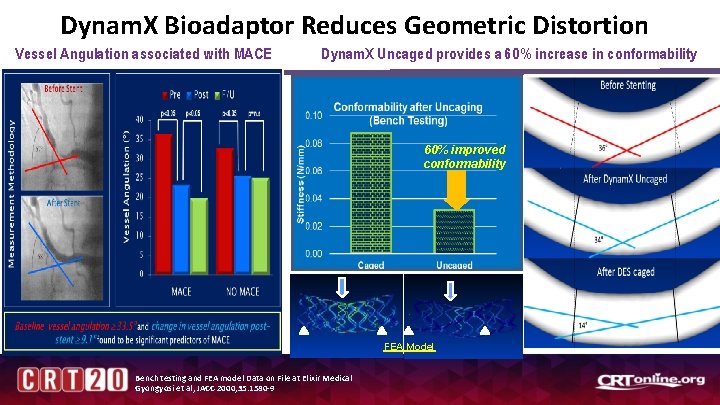
Dynam. X Bioadaptor Reduces Geometric Distortion Vessel Angulation associated with MACE Dynam. X Uncaged provides a 60% increase in conformability 60% improved conformability FEA Model Bench testing and FEA model Data on File at Elixir Medical Gyongyosi et al, JACC 2000; 35: 1580 -9

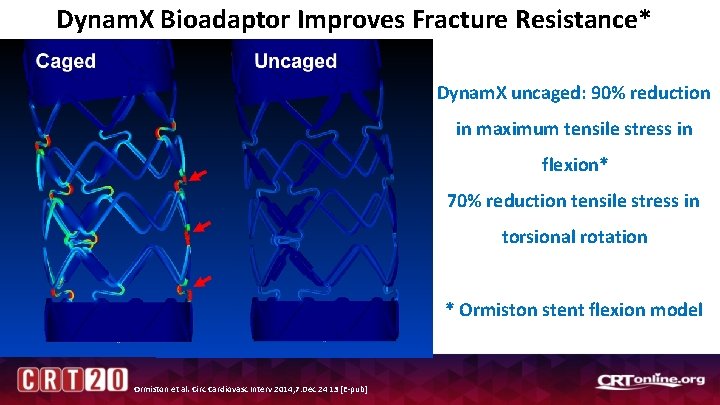
Dynam. X Bioadaptor Improves Fracture Resistance* Dynam. X uncaged: 90% reduction in maximum tensile stress in flexion* 70% reduction tensile stress in torsional rotation * Ormiston stent flexion model Ormiston et al. Circ Cardiovasc Interv 2014; 7: Dec 24 13 [E-pub]

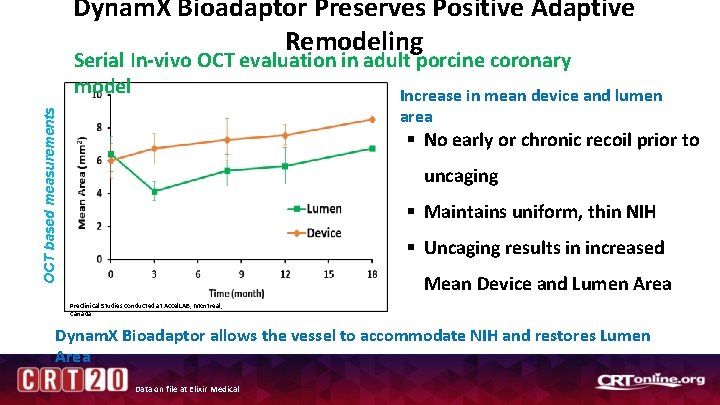
Dynam. X Bioadaptor Preserves Positive Adaptive Remodeling Serial In-vivo OCT evaluation in adult porcine coronary model Increase in mean device and lumen OCT based measurements area § No early or chronic recoil prior to uncaging § Maintains uniform, thin NIH § Uncaging results in increased Mean Device and Lumen Area Preclinical Studies conducted at Accel. LAB, Montreal, Canada Dynam. X Bioadaptor allows the vessel to accommodate NIH and restores Lumen Area Data on file at Elixir Medical

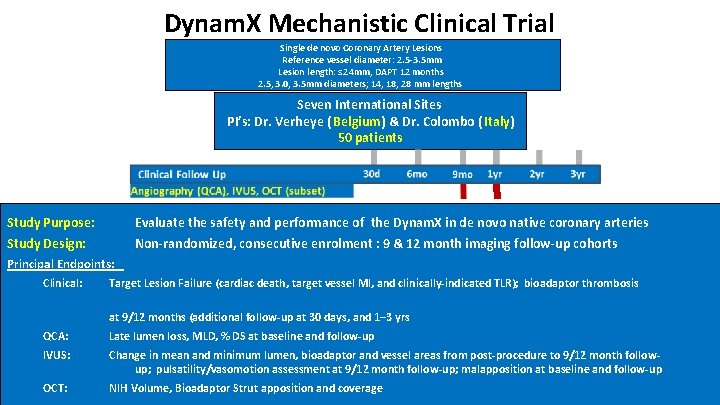
Dynam. X Mechanistic Clinical Trial Single de novo Coronary Artery Lesions Reference vessel diameter: 2. 5 -3. 5 mm Lesion length: ≤ 24 mm, DAPT 12 months 2. 5, 3. 0, 3. 5 mm diameters; 14, 18, 28 mm lengths Seven International Sites PI’s: Dr. Verheye ( Belgium) & Dr. Colombo ( Italy) 50 patients Study Purpose: Evaluate the safety and performance of the Dynam. X in de novo native coronary arteries Study Design: Non-randomized, consecutive enrolment : 9 & 12 month imaging follow-up cohorts Principal Endpoints: Clinical: Target Lesion Failure (cardiac death, target vessel MI, and clinically-indicated TLR); bioadaptor thrombosis at 9/12 months (additional follow-up at 30 days, and 1– 3 yrs QCA: Late lumen loss, MLD, % DS at baseline and follow-up IVUS: Change in mean and minimum lumen, bioadaptor and vessel areas from post-procedure to 9/12 month followup; pulsatility/vasomotion assessment at 9/12 month follow-up; malapposition at baseline and follow-up OCT: NIH Volume, Bioadaptor Strut apposition and coverage

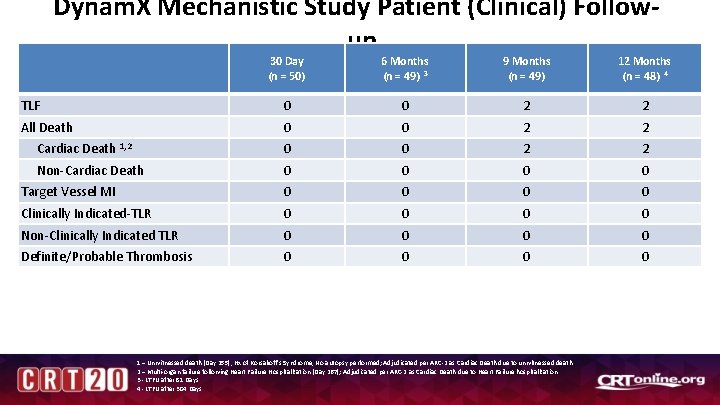
Dynam. X Mechanistic Study Patient (Clinical) Followup 30 Day (n = 50) 6 Months (n = 49) 3 9 Months (n = 49) 12 Months (n = 48) 4 TLF 0 0 2 2 All Death 0 0 2 2 Cardiac Death 1, 2 0 0 2 2 Non-Cardiac Death 0 0 Target Vessel MI 0 0 Clinically Indicated-TLR 0 0 Non-Clinically Indicated TLR 0 0 Definite/Probable Thrombosis 0 0 1 – Unwitnessed death (Day 255), Hx of Korsakoff’s Syndrome, No autopsy performed; Adjudicated per ARC-2 as Cardiac Death due to unwitnessed death. 2 – Multi-organ failure following Heart Failure Hospitalization (Day 267); Adjudicated per ARC-2 as Cardiac Death due to Heart Failure hospitalization. 3 - LTFU after 81 Days 4 - LTFU after 304 Days

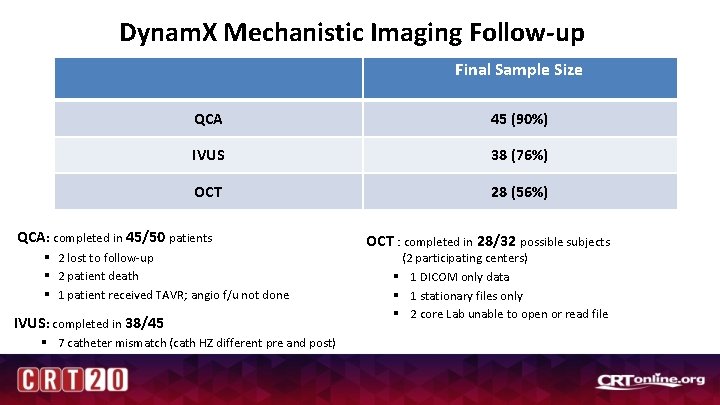
Dynam. X Mechanistic Imaging Follow-up Final Sample Size QCA 45 (90%) IVUS 38 (76%) OCT 28 (56%) QCA: completed in 45/50 patients § 2 lost to follow-up § 2 patient death § 1 patient received TAVR; angio f/u not done IVUS: completed in 38/45 § 7 catheter mismatch (cath HZ different pre and post) OCT : completed in 28/32 possible subjects (2 participating centers) § 1 DICOM only data § 1 stationary files only § 2 core Lab unable to open or read file

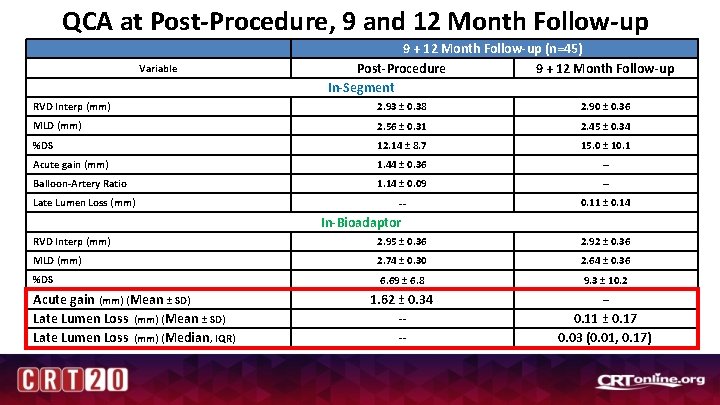
QCA at Post-Procedure, 9 and 12 Month Follow-up Variable 9 + 12 Month Follow-up (n=45) Post-Procedure 9 + 12 Month Follow-up In-Segment RVD Interp (mm) 2. 93 ± 0. 38 2. 90 ± 0. 36 MLD (mm) 2. 56 ± 0. 31 2. 45 ± 0. 34 %DS 12. 14 ± 8. 7 15. 0 ± 10. 1 Acute gain (mm) 1. 44 ± 0. 36 -- Balloon-Artery Ratio 1. 14 ± 0. 09 -- -- 0. 11 ± 0. 14 Late Lumen Loss (mm) In-Bioadaptor RVD Interp (mm) 2. 95 ± 0. 36 2. 92 ± 0. 36 MLD (mm) 2. 74 ± 0. 30 2. 64 ± 0. 36 %DS 6. 69 ± 6. 8 9. 3 ± 10. 2 1. 62 ± 0. 34 -- -- 0. 11 ± 0. 17 0. 03 (0. 01, 0. 17) Acute gain (mm) (Mean ± SD) Late Lumen Loss (mm) (Median , IQR) --

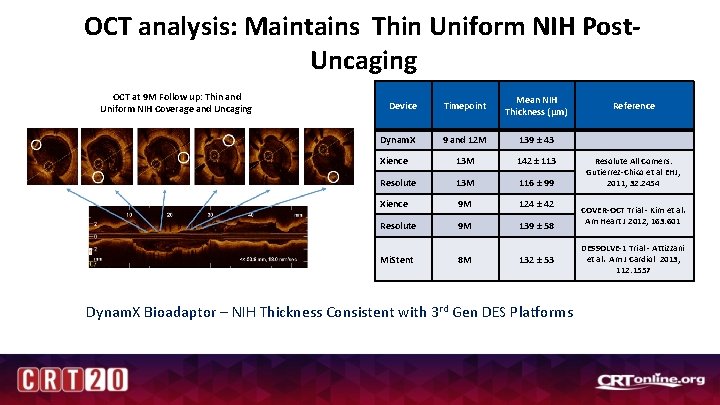
OCT analysis: Maintains Thin Uniform NIH Post. Uncaging OCT at 9 M Follow up: Thin and Uniform NIH Coverage and Uncaging Device Timepoint Mean NIH Thickness (µm) Dynam. X 9 and 12 M 139 ± 43 Xience 13 M 142 ± 113 Resolute 13 M 116 ± 99 Xience 9 M 124 ± 42 Resolute 9 M 139 ± 58 Mi. Stent 8 M 132 ± 53 Dynam. X Bioadaptor – NIH Thickness Consistent with 3 rd Gen DES Platforms Reference Resolute All Comers: Gutierrez-Chico et al EHJ, 2011; 32: 2454 COVER-OCT Trial - Kim et al. Am Heart J 2012; 163: 601 DESSOLVE-1 Trial - Attizzani et al. Am J Cardiol 2013; 112: 1557

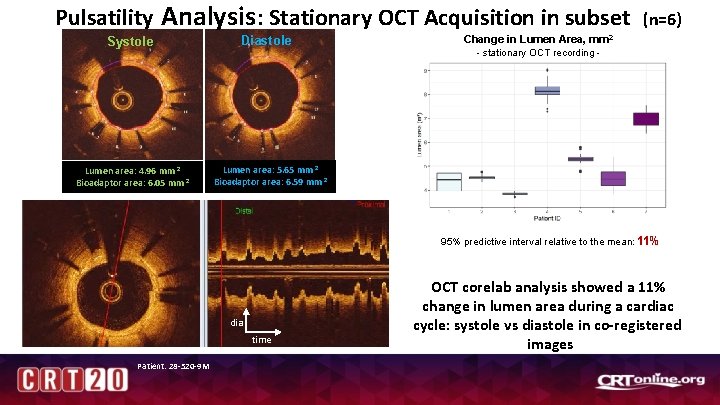
Pulsatility Analysis : Stationary OCT Acquisition in subset Systole Lumen area: 4. 96 mm 2 Bioadaptor area: 6. 05 mm 2 Diastole Change in Lumen Area, (n=6) mm 2 - stationary OCT recording - Lumen area: 5. 65 mm 2 Bioadaptor area: 6. 59 mm 2 95% predictive interval relative to the mean: 11% dia time Patient: 28 -520 -9 M OCT corelab analysis showed a 11% change in lumen area during a cardiac cycle: systole vs diastole in co-registered images

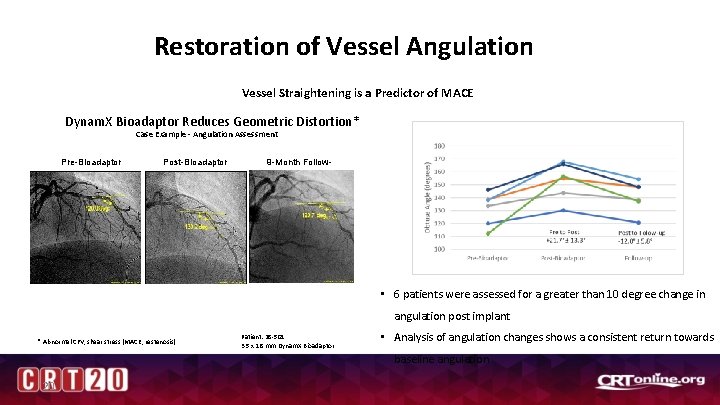
Restoration of Vessel Angulation Vessel Straightening is a Predictor of MACE Dynam. X Bioadaptor Reduces Geometric Distortion* Case Example - Angulation Assessment Pre-Bioadaptor Post-Bioadaptor 9 -Month Followup • 6 patients were assessed for a greater than 10 degree change in angulation post implant * Abnormal CFV, shear stress (MACE, restenosis) Patient: 28 -501 3. 5 x 18 mm Dynam. X Bioadaptor • Analysis of angulation changes shows a consistent return towards baseline angulation 20

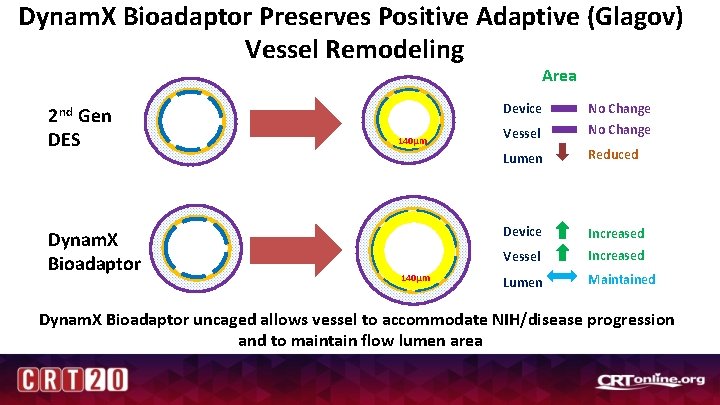
Dynam. X Bioadaptor Preserves Positive Adaptive (Glagov) Vessel Remodeling Area 2 nd Gen DES Dynam. X Bioadaptor Device 140µm Vessel No Change Lumen Reduced Device Increased Vessel Increased Lumen Maintained Dynam. X Bioadaptor uncaged allows vessel to accommodate NIH/disease progression and to maintain flow lumen area

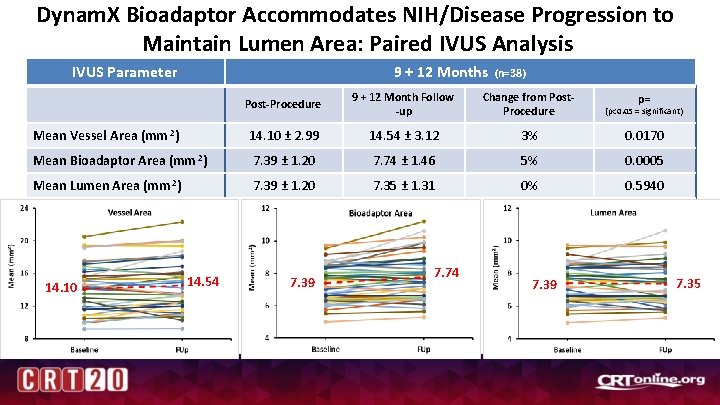
Dynam. X Bioadaptor Accommodates NIH/Disease Progression to Maintain Lumen Area: Paired IVUS Analysis 9 + 12 Months IVUS Parameter (n=38) Post-Procedure 9 + 12 Month Follow -up Change from Post. Procedure (p<0. 05 = significant) Mean Vessel Area (mm 2) 14. 10 ± 2. 99 14. 54 ± 3. 12 3% 0. 0170 Mean Bioadaptor Area (mm 2) 7. 39 ± 1. 20 7. 74 ± 1. 46 5% 0. 0005 Mean Lumen Area (mm 2) 7. 39 ± 1. 20 7. 35 ± 1. 31 0% 0. 5940 14. 10 14. 54 7. 39 7. 74 7. 39 p= 7. 35

Dynam. X Bioadaptor Conclusions • Dynam. X Bioadaptor performs similar to 2 nd gen DES - implantation technique, deliverability, conformability and radial strength while the vessel heals • Innovative uncaging of Dynam. X Bioadaptor restores pulsatility of the vessel and allows the vessel to return towards baseline angulation. • Dynam. X Bioadaptor effectively uncages the vessel to preserve positive adapative remodeling and maintain the vessel lumen - demonstrated by IVUS data and the low QCA LLL at 9/12 months • Dynam. X Bioadaptor demonstrated excellent safety over 9 and 12 month follow-up

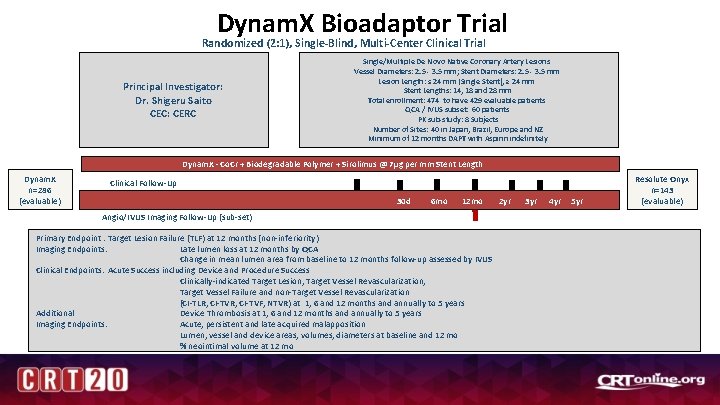
Dynam. X Bioadaptor Trial Randomized (2: 1), Single-Blind, Multi-Center Clinical Trial Principal Investigator: Dr. Shigeru Saito CEC: CERC Single/Multiple De Novo Native Coronary Artery Lesions Vessel Diameters: 2. 5 - 3. 5 mm; Stent Diameters: 2. 5 - 3. 5 mm Lesion Length: ≤ 24 mm (Single Stent), ≥ 24 mm Stent Lengths: 14, 18 and 28 mm Total enrollment: 474 to have 429 evaluable patients QCA / IVUS subset: 60 patients PK sub-study: 8 Subjects Number of Sites: 40 in Japan, Brazil, Europe and NZ Minimum of 12 months DAPT with Aspirin indefinitely Dynam. X - Co. Cr + Biodegradable Polymer + Sirolimus @ 7µg per mm Stent Length Dynam. X n=286 (evaluable) Clinical Follow-Up 30 d 6 mo 12 mo Angio/IVUS Imaging Follow-Up (sub-set) Primary Endpoint : Target Lesion Failure (TLF) at 12 months (non-inferiority) Imaging Endpoints: Late lumen loss at 12 months by QCA Change in mean lumen area from baseline to 12 months follow-up assessed by IVUS Clinical Endpoints: Acute Success including Device and Procedure Success Clinically-indicated Target Lesion, Target Vessel Revascularization, Target Vessel Failure and non-Target Vessel Revascularization (CI-TLR, CI-TVR; CI-TVF; NTVR) at 1, 6 and 12 months and annually to 5 years Additional Device Thrombosis at 1, 6 and 12 months and annually to 5 years Imaging Endpoints: Acute, persistent and late acquired malapposition Lumen, vessel and device areas, volumes, diameters at baseline and 12 mo % neointimal volume at 12 mo 2 yr 3 yr 4 yr 5 yr Resolute Onyx n=143 (evaluable)

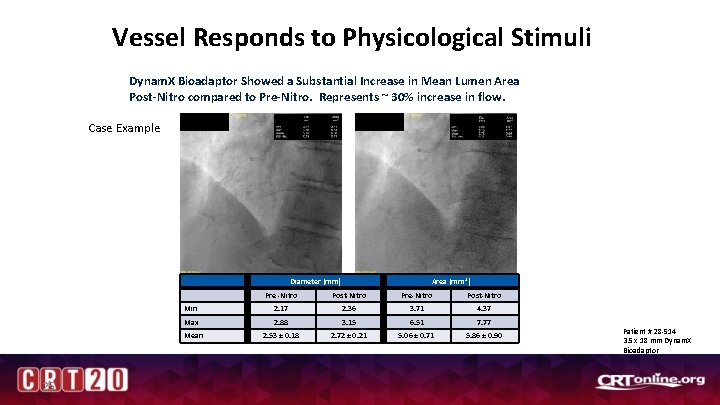
Vessel Responds to Physicological Stimuli Dynam. X Bioadaptor Showed a Substantial Increase in Mean Lumen Area Post-Nitro compared to Pre-Nitro. Represents ~ 30% increase in flow. Case Example Pre-Nitro Post-Nitro Diameter (mm) Min 25 Area (mm 2) Pre -Nitro Post-Nitro Pre-Nitro Post-Nitro 2. 17 2. 36 3. 71 4. 37 Max 2. 88 3. 15 6. 51 7. 77 Mean 2. 53 ± 0. 18 2. 72 ± 0. 21 5. 06 ± 0. 71 5. 86 ± 0. 90 Patient # 28 -514 3. 5 x 18 mm Dynam. X Bioadaptor