Dnyanasadhana College Thane Department of Chemistry T Y







- Slides: 37

Dnyanasadhana College, Thane. Department of Chemistry T. Y. B. Sc. Analytical Chemistry Paper-IV Sem-V Atomic Absorption Spectroscopy Dr. G. R. Bhagure 1

Atomic Absorption Spectroscopy UNIT-IV 2

Introduction • In Analytical chemistry Atomic absorption spectroscopy is a technique for determining the concentration of a particular metal element in a sample. Atomic absorption spectroscopy can be used to analyze the concentration of over 62 different metals in a solution. 3

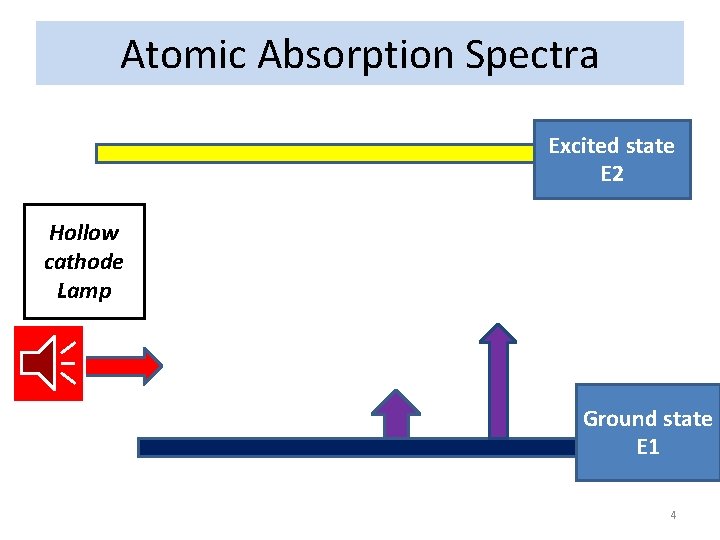
Atomic Absorption Spectra Excited state E 2 Hollow cathode Lamp Ground state E 1 4

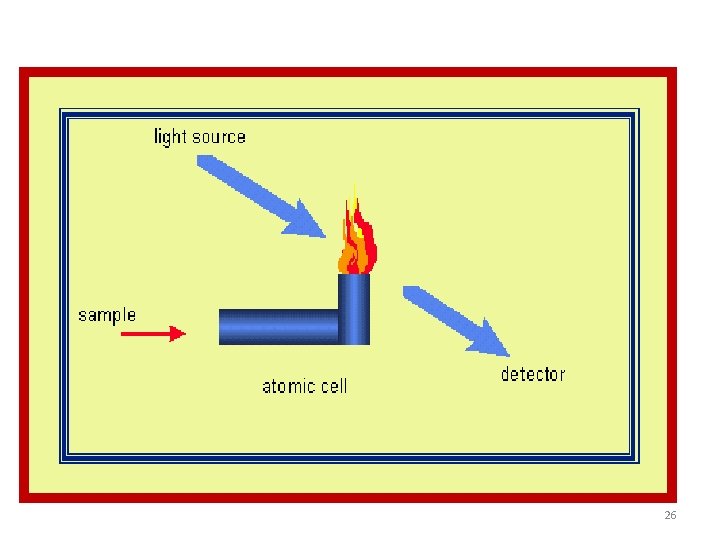
Technique • The technique typically makes use of a flame to atomize the sample, but other atomizers such as a graphite furnace are also used. • Three steps are involved in turning a liquid sample into an atomic gas: 5

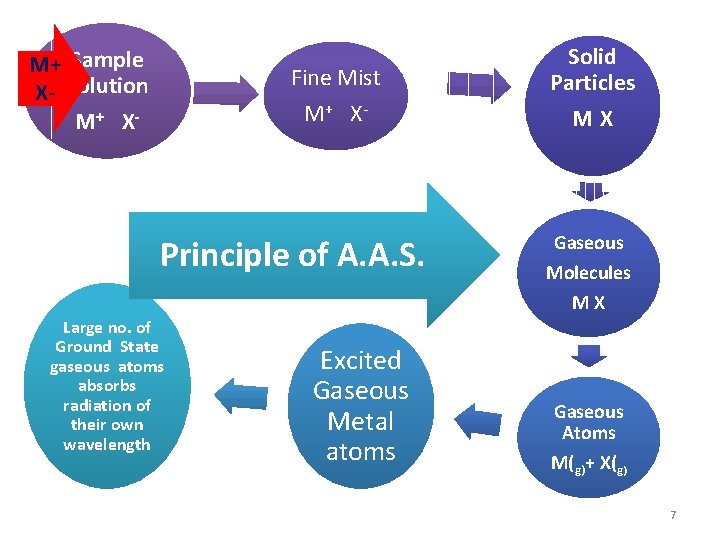
Three steps In AAS § § Three steps are involved in turning a liquid sample into an atomic gas: Desolvation – the liquid solvent is evaporated and the dry sample remains Vaporization – the solid sample vaporizes to a gas Volatilization – the compounds making up the sample are broken into free atoms 6

M+ Sample X- Solution M+ X - Fine Mist M + X- Principle of A. A. S. Principle of F. E. S Solid Particles MX Gaseous Molecules MX Large no. of Ground State gaseous atoms absorbs radiation of their own wavelength Excited Gaseous Metal atoms Gaseous Atoms M(g)+ X(g) 7

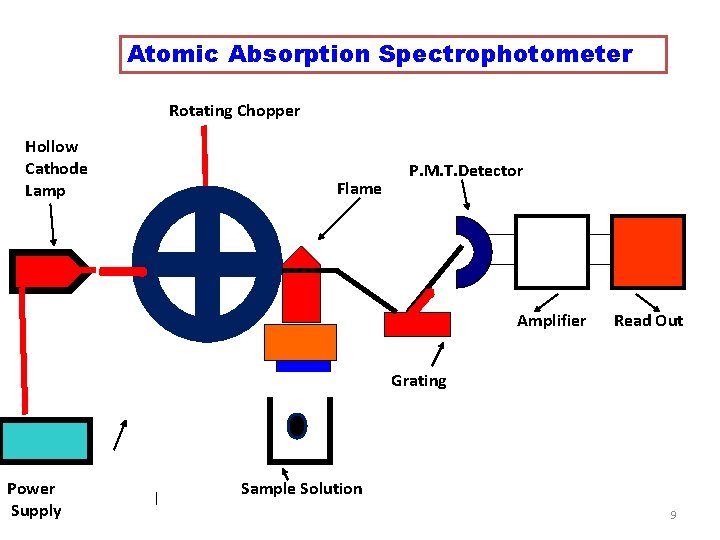
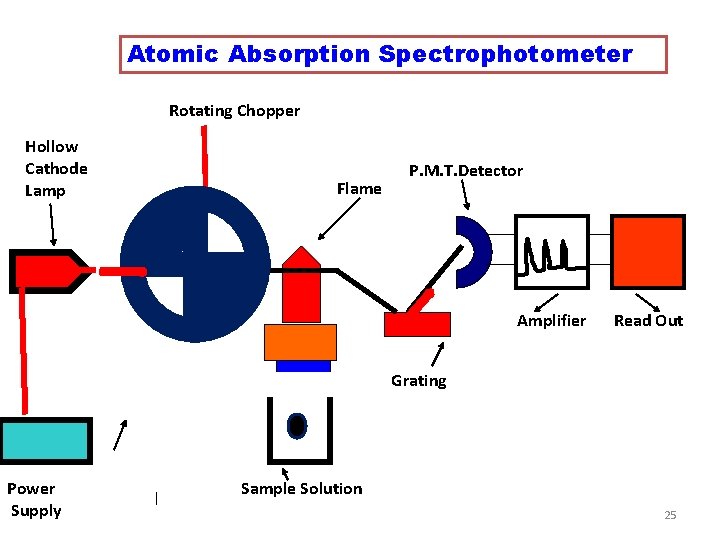
Instrumentation • The Instrument used in AAS is called as ATOMIC ABSORPTION SPECTROPHOTOMETER The Components are 1. Radiation Source : Hollow Cathode Lamp 2. Rotating Chopper 3. Atomisation Unit 4. Monochromator 5. Detector and Amplifier 6. Read out device 8

Atomic Absorption Spectrophotometer Rotating Chopper Hollow Cathode Lamp Flame P. M. T. Detector Amplifier Read Out Grating Power Supply Sample Solution 9

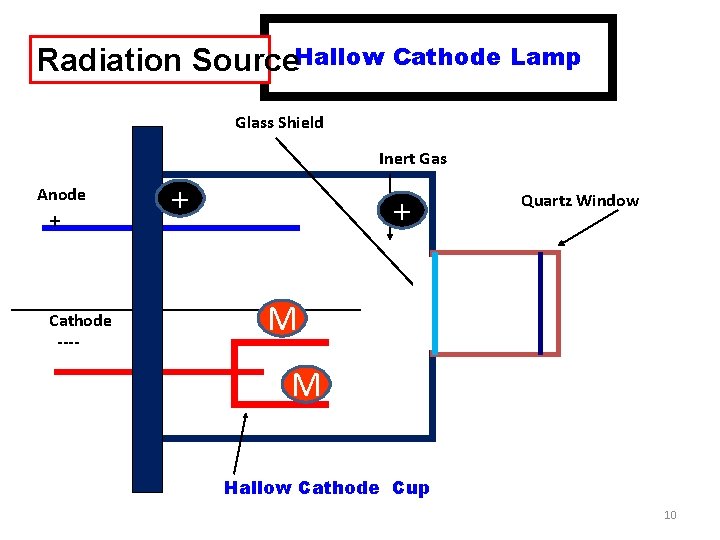
Radiation Source. Hallow Cathode Lamp Glass Shield Inert Gas Anode + + + Quartz Window ____________________ Cathode ---- M M Hallow Cathode Cup 10

11

12

13

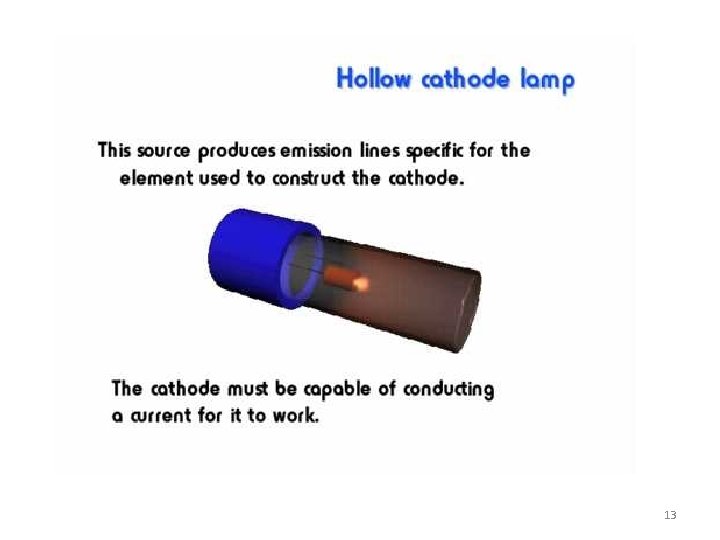
Radiation Source : • Radiation Source : Hollow Cathode Lamp • HCL is the most common radiation source in atomic absorption spectroscopy. The lamp consist of glass tube. The Lamp is filled with argon or neon gas. The cathode is a cylindrical metal cathode containing the metal for excitation, and an anode. When a high voltage is applied across the anode and cathode, gas particles are ionized. As voltage is increased, gaseous ions acquire enough energy to eject metal atoms from the cathode. Some of these atoms are in excited states and emit light with the frequency characteristic to the metal. Many modern hollow cathode lamps are selective for several metals. 14

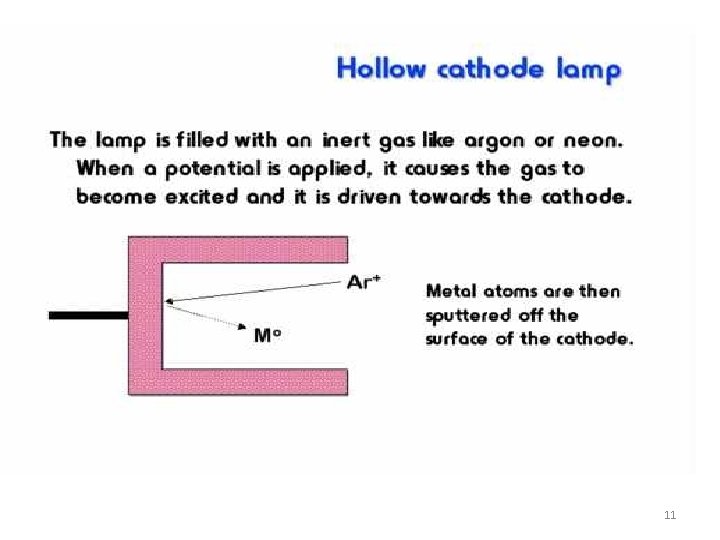
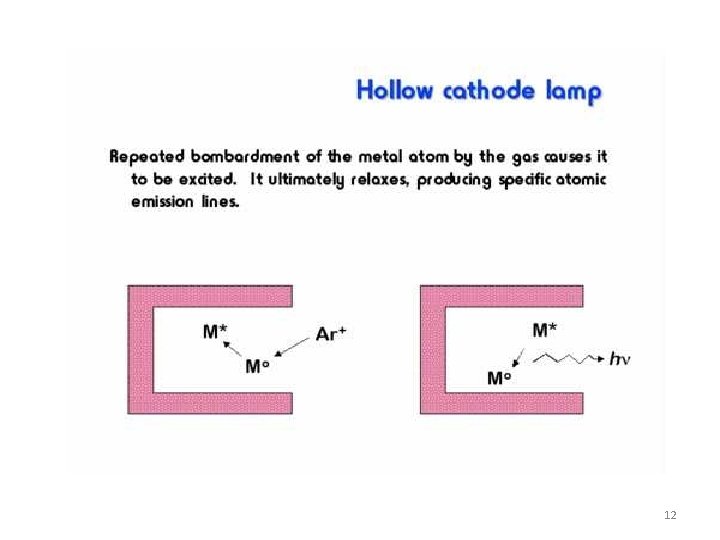
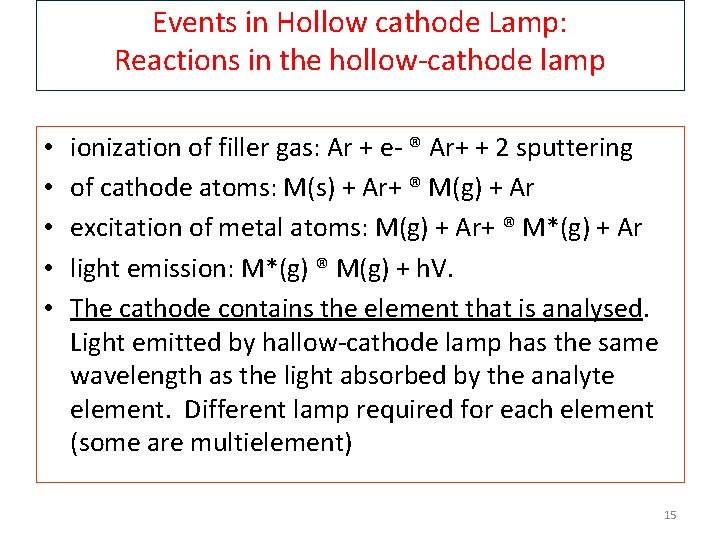
Events in Hollow cathode Lamp: Reactions in the hollow-cathode lamp • • • ionization of filler gas: Ar + e- ® Ar+ + 2 sputtering of cathode atoms: M(s) + Ar+ ® M(g) + Ar excitation of metal atoms: M(g) + Ar+ ® M*(g) + Ar light emission: M*(g) ® M(g) + h. V. The cathode contains the element that is analysed. Light emitted by hallow-cathode lamp has the same wavelength as the light absorbed by the analyte element. Different lamp required for each element (some are multielement) 15

• The type of hollow cathode tube depends on the metal being analyzed. For analyzing the concentration of copper in an ore, a copper cathode tube would be used, and likewise for any other metal being analyzed. The electrons of the atoms in the flame can be promoted to higher orbitals for an instant by absorbing a set quantity of energy (a quantum). 16

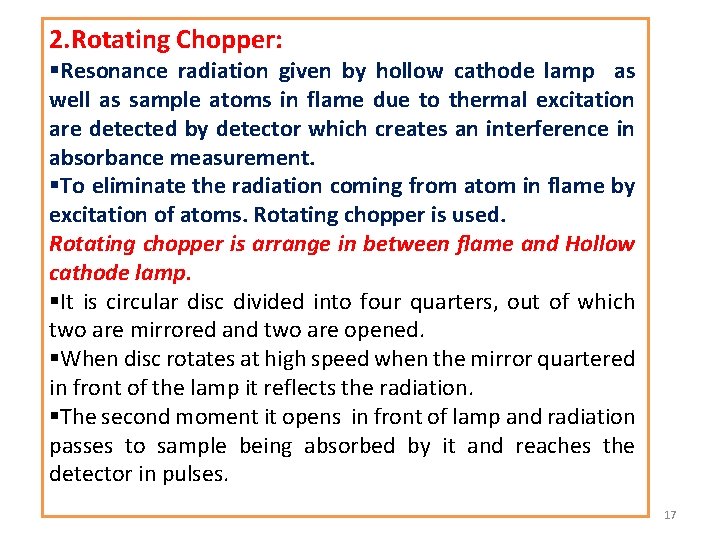
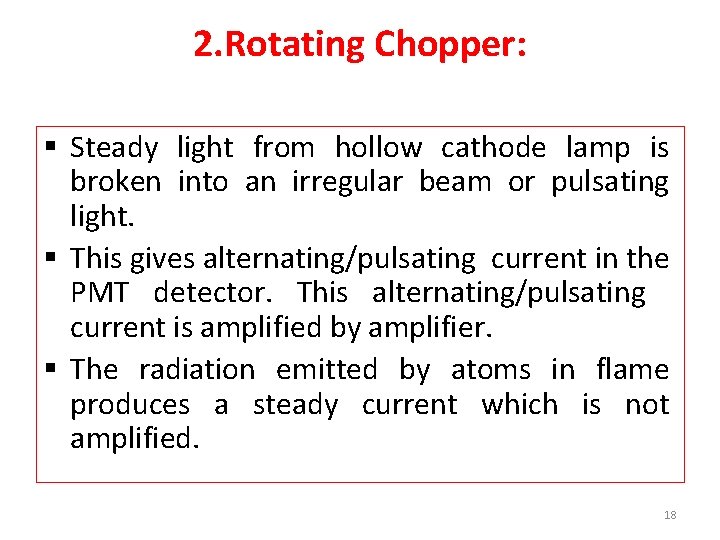
2. Rotating Chopper: §Resonance radiation given by hollow cathode lamp as well as sample atoms in flame due to thermal excitation are detected by detector which creates an interference in absorbance measurement. §To eliminate the radiation coming from atom in flame by excitation of atoms. Rotating chopper is used. Rotating chopper is arrange in between flame and Hollow cathode lamp. §It is circular disc divided into four quarters, out of which two are mirrored and two are opened. §When disc rotates at high speed when the mirror quartered in front of the lamp it reflects the radiation. §The second moment it opens in front of lamp and radiation passes to sample being absorbed by it and reaches the detector in pulses. 17

2. Rotating Chopper: § Steady light from hollow cathode lamp is broken into an irregular beam or pulsating light. § This gives alternating/pulsating current in the PMT detector. This alternating/pulsating current is amplified by amplifier. § The radiation emitted by atoms in flame produces a steady current which is not amplified. 18

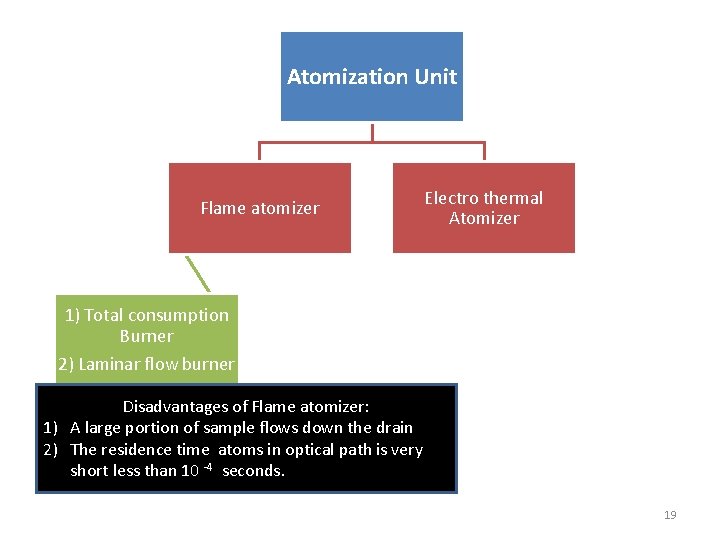
Atomization Unit Flame atomizer Electro thermal Atomizer 1) Total consumption Burner 2) Laminar flow burner Disadvantages of Flame atomizer: 1) A large portion of sample flows down the drain 2) The residence time atoms in optical path is very short less than 10 -4 seconds. 19

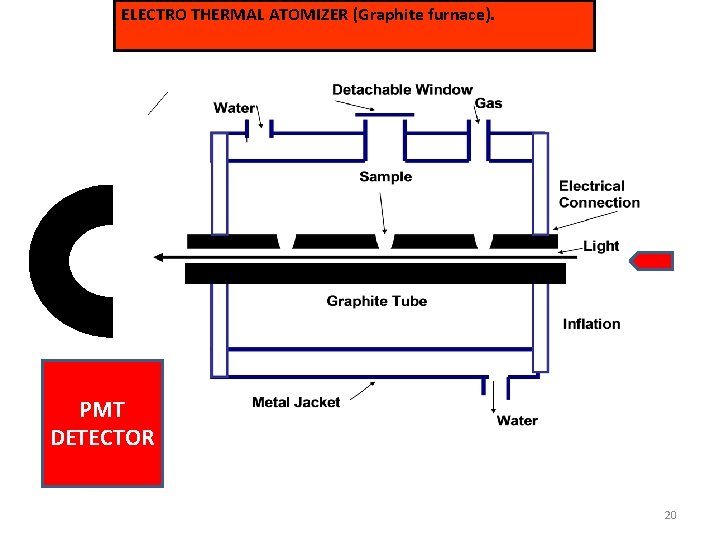
ELECTRO THERMAL ATOMIZER (Graphite furnace). PMT DETECTOR 20

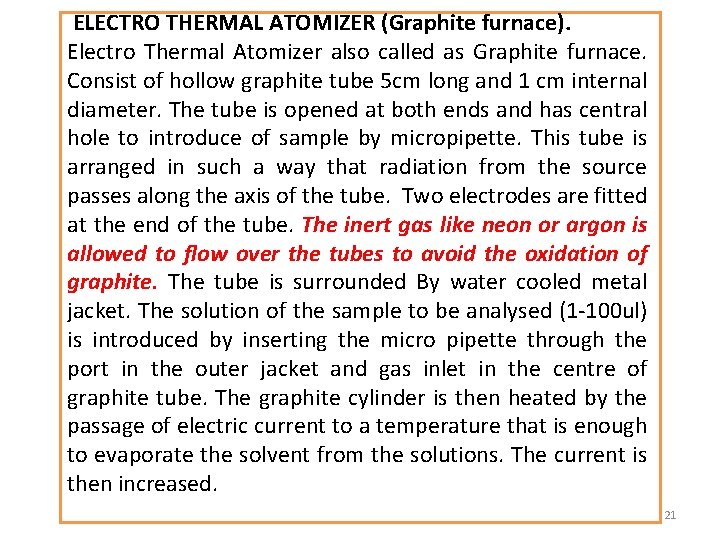
ELECTRO THERMAL ATOMIZER (Graphite furnace). Electro Thermal Atomizer also called as Graphite furnace. Consist of hollow graphite tube 5 cm long and 1 cm internal diameter. The tube is opened at both ends and has central hole to introduce of sample by micropipette. This tube is arranged in such a way that radiation from the source passes along the axis of the tube. Two electrodes are fitted at the end of the tube. The inert gas like neon or argon is allowed to flow over the tubes to avoid the oxidation of graphite. The tube is surrounded By water cooled metal jacket. The solution of the sample to be analysed (1 -100 ul) is introduced by inserting the micro pipette through the port in the outer jacket and gas inlet in the centre of graphite tube. The graphite cylinder is then heated by the passage of electric current to a temperature that is enough to evaporate the solvent from the solutions. The current is then increased. 21

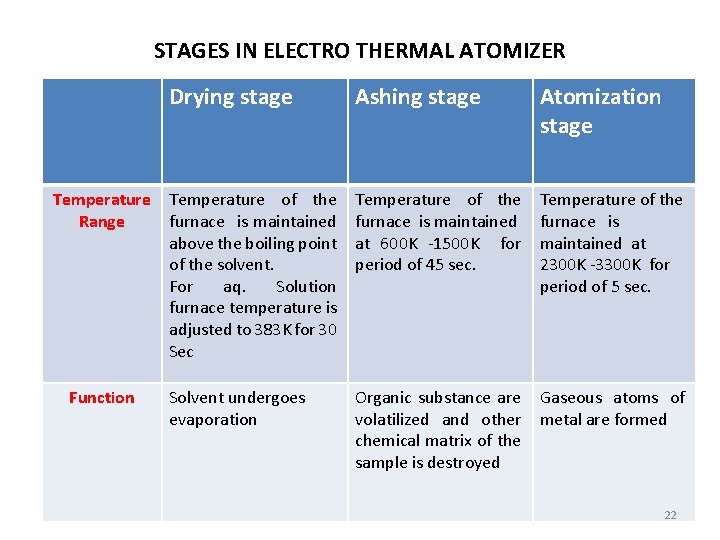
STAGES IN ELECTRO THERMAL ATOMIZER Temperature Range Function Drying stage Ashing stage Atomization stage Temperature of the furnace is maintained above the boiling point of the solvent. For aq. Solution furnace temperature is adjusted to 383 K for 30 Sec Temperature of the furnace is maintained at 600 K -1500 K for period of 45 sec. Temperature of the furnace is maintained at 2300 K -3300 K for period of 5 sec. Solvent undergoes evaporation Organic substance are volatilized and other chemical matrix of the sample is destroyed Gaseous atoms of metal are formed 22

Advantages: §Temperature that is enough to ash the sample and to produce the metal atoms. § The sample volume is small § No need for fuel -oxidant mixture. §High sensitivity §No flame noise. § Solid sample can be used directly. §Heat distribution is uniform and temperature is steady. 23

4) Monochromator: Prism or grating monochromator are used in AAS. The function of the monochromator is to select a given absorbing line from spectral lines by emitting hallow cathode lamp. 24

Atomic Absorption Spectrophotometer Rotating Chopper Hollow Cathode Lamp Flame P. M. T. Detector Amplifier Read Out Grating Power Supply Sample Solution 25

26

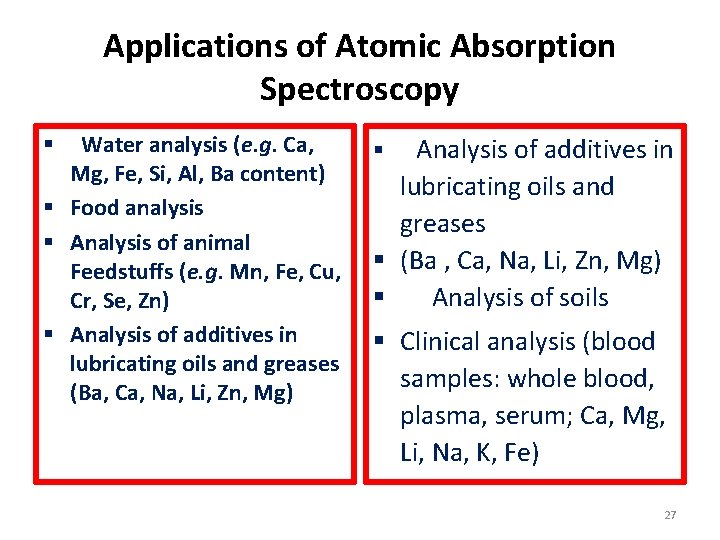
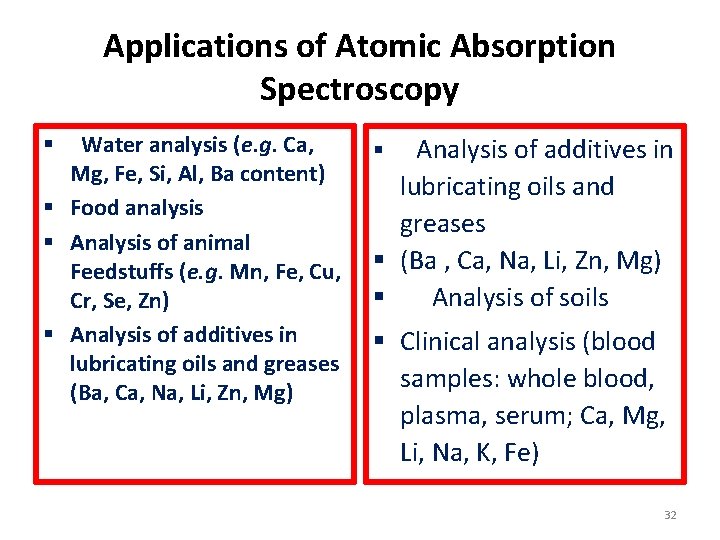
Applications of Atomic Absorption Spectroscopy Water analysis (e. g. Ca, Mg, Fe, Si, Al, Ba content) § Food analysis § Analysis of animal Feedstuffs (e. g. Mn, Fe, Cu, Cr, Se, Zn) § Analysis of additives in lubricating oils and greases (Ba, Ca, Na, Li, Zn, Mg) § Analysis of additives in lubricating oils and greases § (Ba , Ca, Na, Li, Zn, Mg) § Analysis of soils § § Clinical analysis (blood samples: whole blood, plasma, serum; Ca, Mg, Li, Na, K, Fe) 27

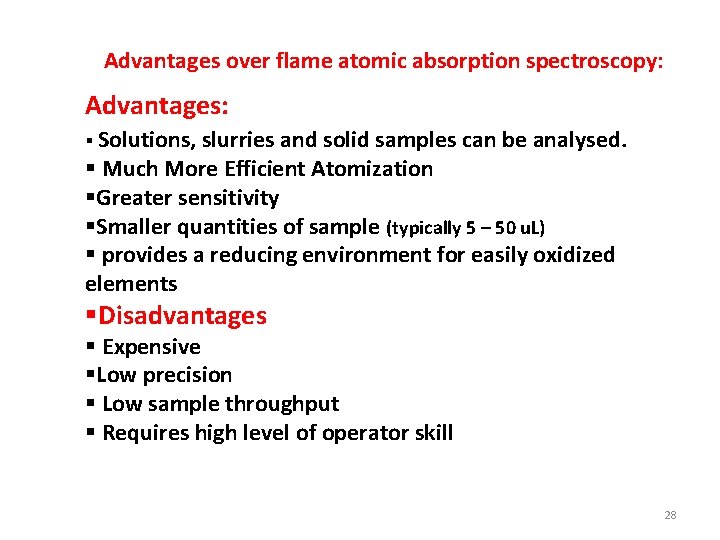
Advantages over flame atomic absorption spectroscopy: Advantages: § Solutions, slurries and solid samples can be analysed. § Much More Efficient Atomization §Greater sensitivity §Smaller quantities of sample (typically 5 – 50 u. L) § provides a reducing environment for easily oxidized elements §Disadvantages § Expensive §Low precision § Low sample throughput § Requires high level of operator skill 28

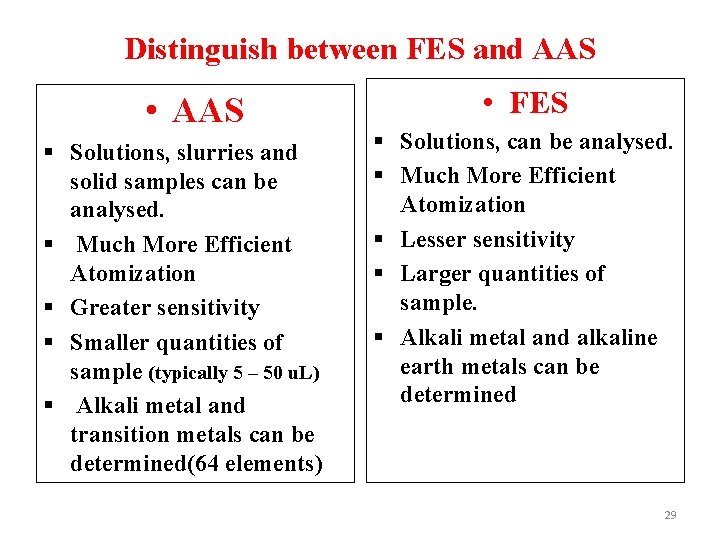
Distinguish between FES and AAS • AAS § Solutions, slurries and solid samples can be analysed. § Much More Efficient Atomization § Greater sensitivity § Smaller quantities of sample (typically 5 – 50 u. L) § Alkali metal and transition metals can be determined(64 elements) • FES § Solutions, can be analysed. § Much More Efficient Atomization § Lesser sensitivity § Larger quantities of sample. § Alkali metal and alkaline earth metals can be determined 29

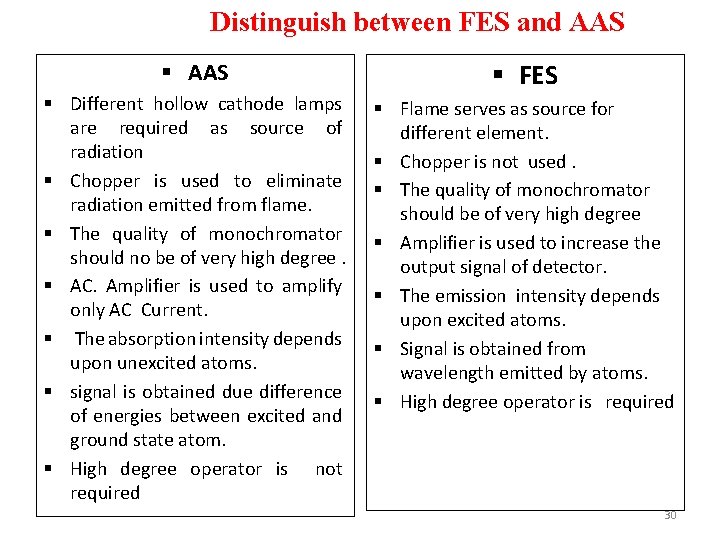
Distinguish between FES and AAS § Different hollow cathode lamps are required as source of radiation § Chopper is used to eliminate radiation emitted from flame. § The quality of monochromator should no be of very high degree. § AC. Amplifier is used to amplify only AC Current. § The absorption intensity depends upon unexcited atoms. § signal is obtained due difference of energies between excited and ground state atom. § High degree operator is not required § FES § Flame serves as source for different element. § Chopper is not used. § The quality of monochromator should be of very high degree § Amplifier is used to increase the output signal of detector. § The emission intensity depends upon excited atoms. § Signal is obtained from wavelength emitted by atoms. § High degree operator is required 30

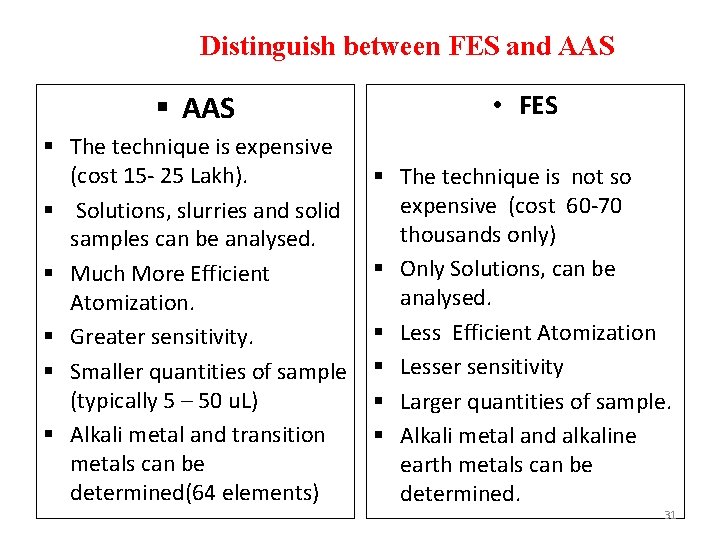
Distinguish between FES and AAS § AAS • FES § The technique is expensive (cost 15 - 25 Lakh). § Solutions, slurries and solid samples can be analysed. § Much More Efficient Atomization. § Greater sensitivity. § Smaller quantities of sample (typically 5 – 50 u. L) § Alkali metal and transition metals can be determined(64 elements) § The technique is not so expensive (cost 60 -70 thousands only) § Only Solutions, can be analysed. § Less Efficient Atomization § Lesser sensitivity § Larger quantities of sample. § Alkali metal and alkaline earth metals can be determined. 31

Applications of Atomic Absorption Spectroscopy Water analysis (e. g. Ca, Mg, Fe, Si, Al, Ba content) § Food analysis § Analysis of animal Feedstuffs (e. g. Mn, Fe, Cu, Cr, Se, Zn) § Analysis of additives in lubricating oils and greases (Ba, Ca, Na, Li, Zn, Mg) § Analysis of additives in lubricating oils and greases § (Ba , Ca, Na, Li, Zn, Mg) § Analysis of soils § § Clinical analysis (blood samples: whole blood, plasma, serum; Ca, Mg, Li, Na, K, Fe) 32

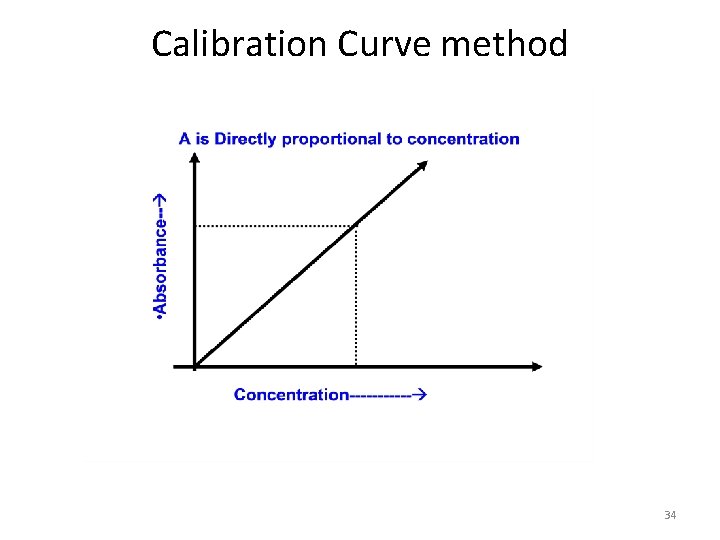
Quantitative Analysis • Calibration Curve method • Standard addition method • Internal Standard addition method 33

Calibration Curve method 34

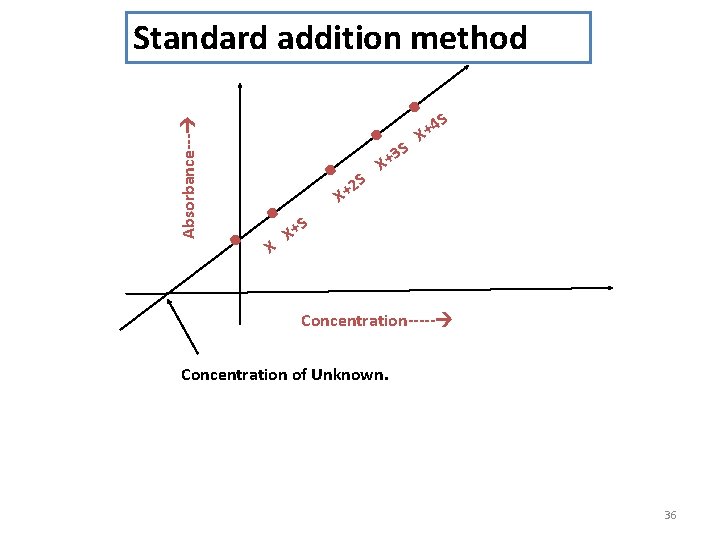
Standard addition method • In this method the absorbance of unknown (X) is first found out by aspirating into flame against blank. Then a series of standards having definite amount of unknown (X) plus varying amount of standard are prepared and diluted to same volume in each case. Their absorbance are then obtained. A graph of absorbance (A) against concentrations of standard (S) gives a linear curve. The concentration of the unknown can be determined by extrapolation of line which cuts to X axis. 35

Absorbance--- Standard addition method . . X . . . S S 2 X+ 3 X+ S 4 X+ S X+ Concentration----- Concentration of Unknown. 36

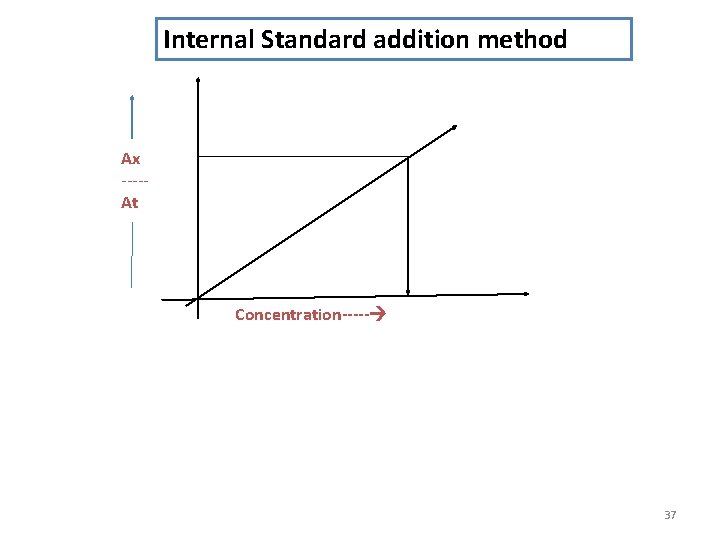
Internal Standard addition method Ax ----At Concentration----- 37
 Satish pradhan dnyanasadhana college
Satish pradhan dnyanasadhana college Satish pradhan dnyanasadhana college online form
Satish pradhan dnyanasadhana college online form Satish pradhan dnyanasadhana college
Satish pradhan dnyanasadhana college Dnyansadhana college thane
Dnyansadhana college thane Satish pradhan college
Satish pradhan college Is macbeth thane of glamis
Is macbeth thane of glamis Trans thane creek industrial area
Trans thane creek industrial area Whos the thane of fife
Whos the thane of fife Usf chemistry
Usf chemistry Nit calicut chemistry faculty
Nit calicut chemistry faculty Manipal university chemistry department
Manipal university chemistry department Texas tech departments
Texas tech departments Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Pasadena city college police department
Pasadena city college police department Brooklyn college organic chemistry
Brooklyn college organic chemistry Excelsior college chemistry
Excelsior college chemistry Asd college college readiness program
Asd college college readiness program Early college high school at midland college
Early college high school at midland college Sterile processing department workflow
Sterile processing department workflow Why strategic planning is important
Why strategic planning is important Uta math
Uta math Swot analysis for procurement department
Swot analysis for procurement department Objective of warehouse
Objective of warehouse Wakulla county recreation department
Wakulla county recreation department Department st laghouat
Department st laghouat Undss security
Undss security The finance department
The finance department Ucl computer science faculty
Ucl computer science faculty Doe inspector general
Doe inspector general Tiffany taylor georgia department of education
Tiffany taylor georgia department of education Function of marketing research
Function of marketing research Operation planning
Operation planning Bloomington indiana police department
Bloomington indiana police department Tsdps rainfall integration
Tsdps rainfall integration Fire department succession planning
Fire department succession planning Rebecca corrigan olmsted falls
Rebecca corrigan olmsted falls Equipment
Equipment