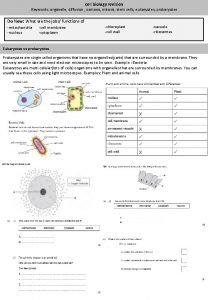
Diffusion of Water Osmosis To survive plants must



- Slides: 18

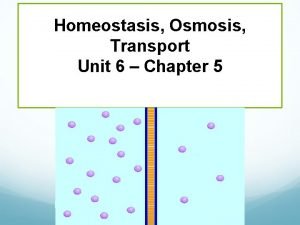
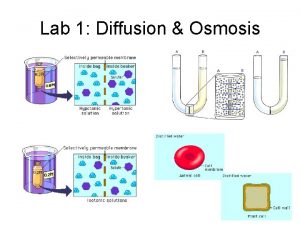
Diffusion of Water (Osmosis) • To survive, plants must balance water uptake and loss • Osmosis determines the net uptake or water loss by a cell and is affected by solute concentration and pressure Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

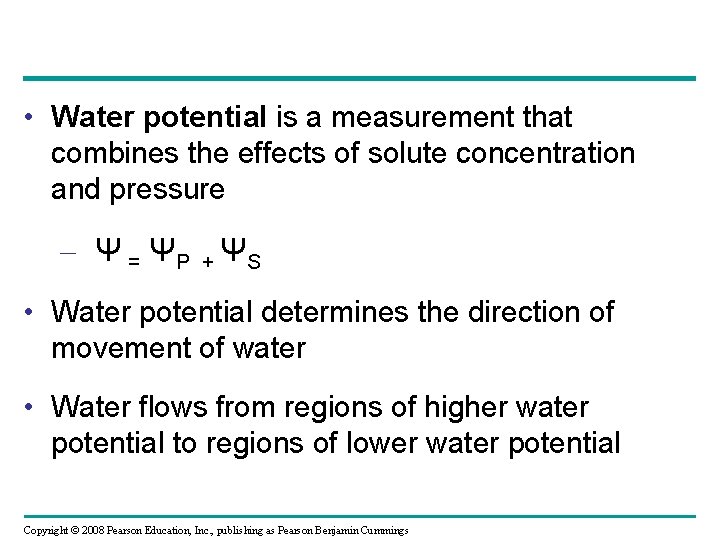
• Water potential is a measurement that combines the effects of solute concentration and pressure – Ψ = ΨP + ΨS • Water potential determines the direction of movement of water • Water flows from regions of higher water potential to regions of lower water potential Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

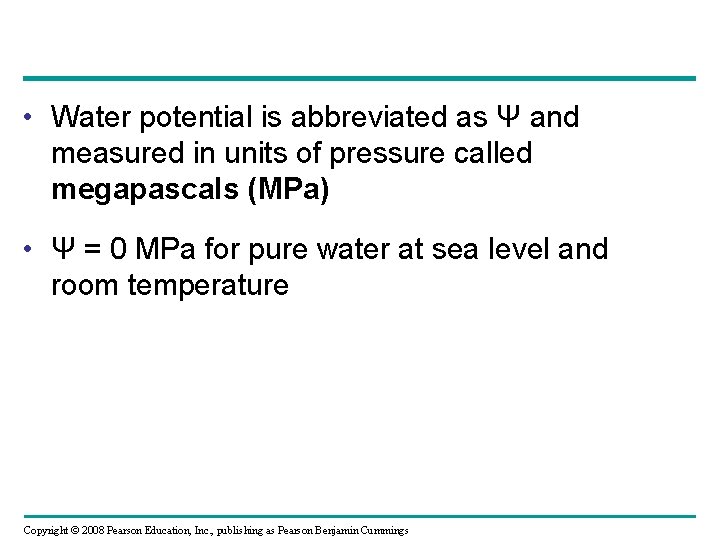
• Water potential is abbreviated as Ψ and measured in units of pressure called megapascals (MPa) • Ψ = 0 MPa for pure water at sea level and room temperature Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

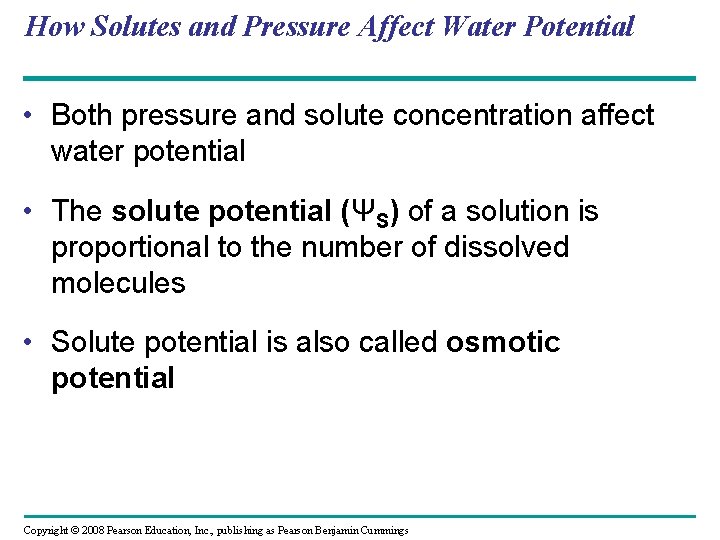
How Solutes and Pressure Affect Water Potential • Both pressure and solute concentration affect water potential • The solute potential (ΨS) of a solution is proportional to the number of dissolved molecules • Solute potential is also called osmotic potential Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

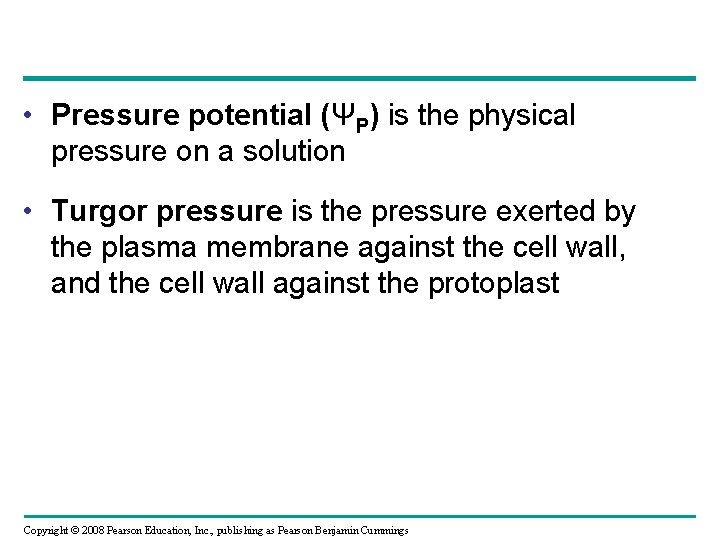
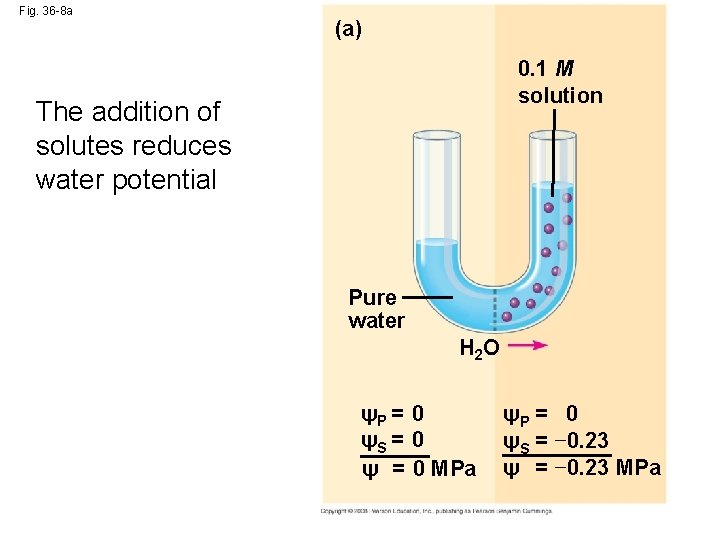
• Pressure potential (ΨP) is the physical pressure on a solution • Turgor pressure is the pressure exerted by the plasma membrane against the cell wall, and the cell wall against the protoplast Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

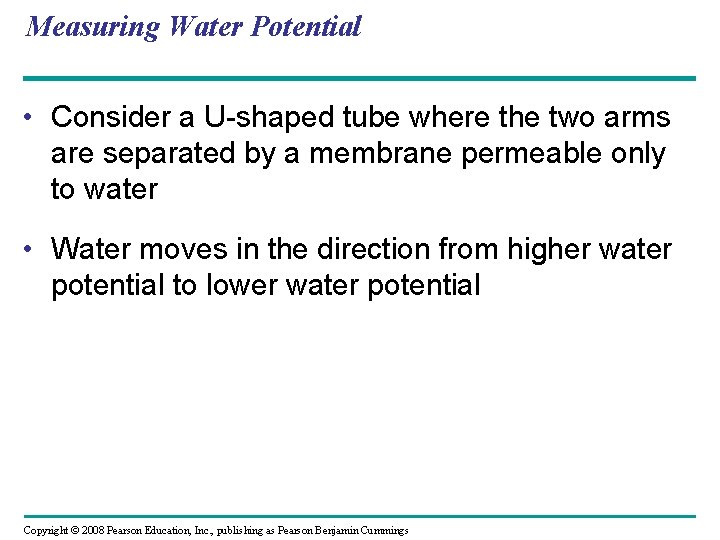
Measuring Water Potential • Consider a U-shaped tube where the two arms are separated by a membrane permeable only to water • Water moves in the direction from higher water potential to lower water potential Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fig. 36 -8 a (a) 0. 1 M solution The addition of solutes reduces water potential Pure water H 2 O ψP = 0 ψS = 0 ψ = 0 MPa ψP = 0 ψS = − 0. 23 ψ = − 0. 23 MPa

Fig. 36 -8 b (b) Positive pressure Physical pressure increases water potential H 2 O ψP = 0 ψS = 0 ψ = 0 MPa ψP = 0. 23 ψS = − 0. 23 ψ = 0 MPa

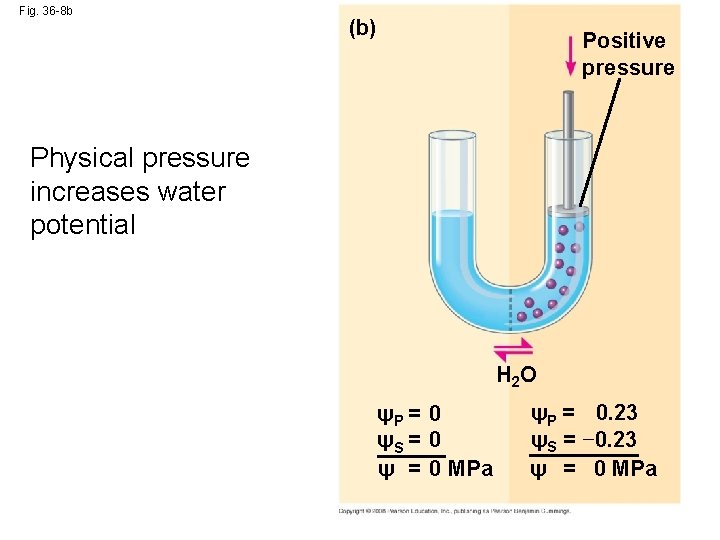
Fig. 36 -8 c (c) Increased positive pressure Further Physical pressure increases water potential more H 2 O ψP = 0. 30 ψP = 0 ψS = − 0. 23 ψS = 0 ψ = 0 MPa ψ = 0. 07 MPa

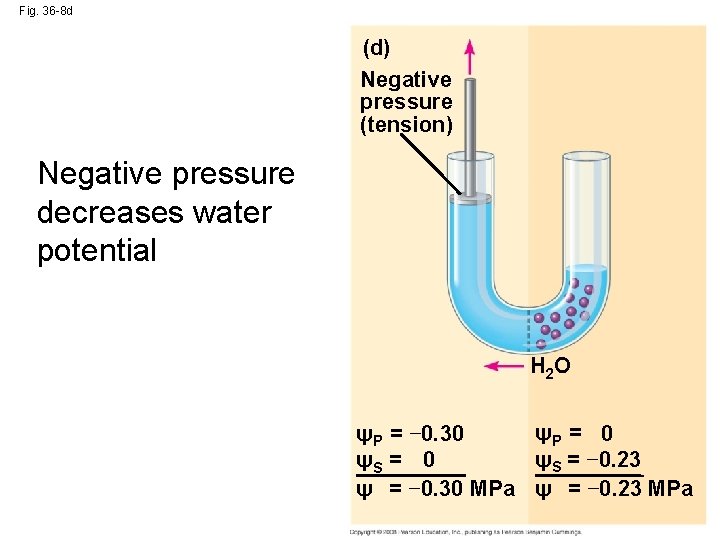
Fig. 36 -8 d (d) Negative pressure (tension) Negative pressure decreases water potential H 2 O ψP = 0 ψP = − 0. 30 ψS = − 0. 23 ψS = 0 ψ = − 0. 30 MPa ψ = − 0. 23 MPa

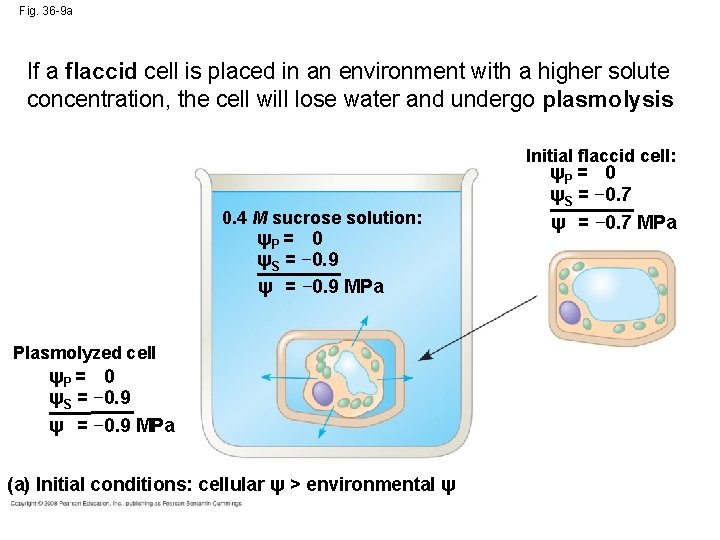
Fig. 36 -9 a If a flaccid cell is placed in an environment with a higher solute concentration, the cell will lose water and undergo plasmolysis Initial flaccid cell: ψP = 0 ψS = − 0. 7 ψ = − 0. 7 MPa 0. 4 M sucrose solution: ψP = 0 ψS = − 0. 9 ψ = − 0. 9 MPa Plasmolyzed cell ψP = 0 ψS = − 0. 9 ψ = − 0. 9 MPa (a) Initial conditions: cellular ψ > environmental ψ

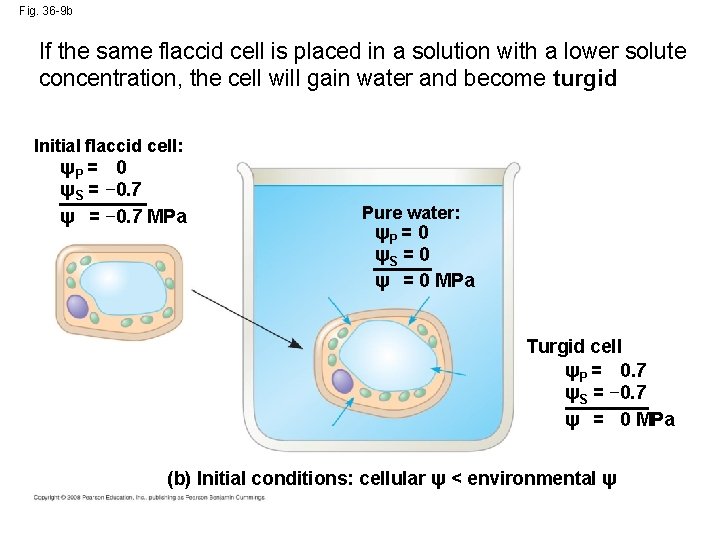
Fig. 36 -9 b If the same flaccid cell is placed in a solution with a lower solute concentration, the cell will gain water and become turgid Initial flaccid cell: ψP = 0 ψS = − 0. 7 ψ = − 0. 7 MPa Pure water: ψP = 0 ψS = 0 ψ = 0 MPa Turgid cell ψP = 0. 7 ψS = − 0. 7 ψ = 0 MPa (b) Initial conditions: cellular ψ < environmental ψ

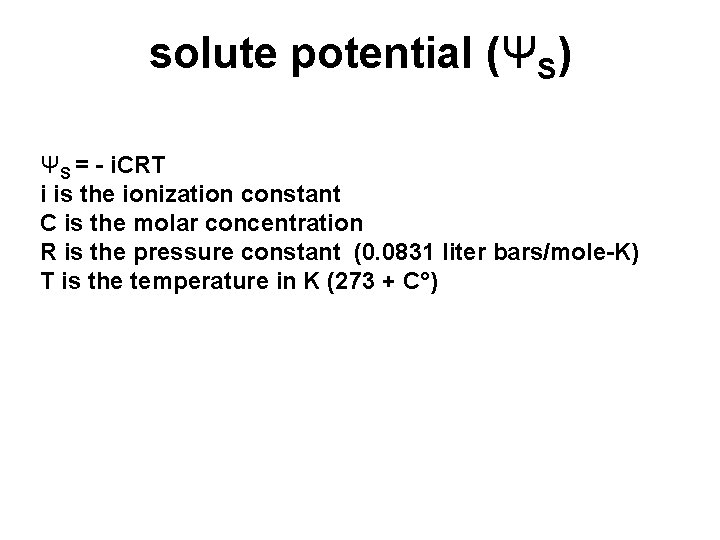
solute potential (ΨS) ΨS = - i. CRT i is the ionization constant C is the molar concentration R is the pressure constant (0. 0831 liter bars/mole-K) T is the temperature in K (273 + C°)

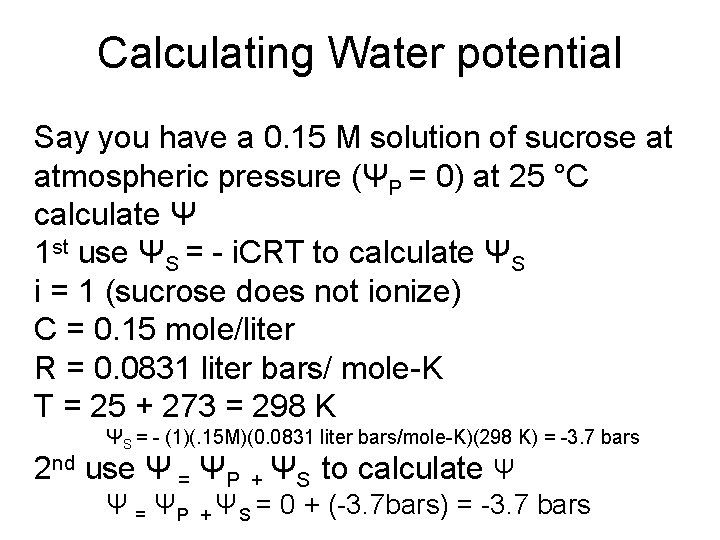
Calculating Water potential Say you have a 0. 15 M solution of sucrose at atmospheric pressure (ΨP = 0) at 25 °C calculate Ψ 1 st use ΨS = - i. CRT to calculate ΨS i = 1 (sucrose does not ionize) C = 0. 15 mole/liter R = 0. 0831 liter bars/ mole-K T = 25 + 273 = 298 K ΨS = - (1)(. 15 M)(0. 0831 liter bars/mole-K)(298 K) = -3. 7 bars 2 nd use Ψ = ΨP + ΨS to calculate Ψ Ψ = ΨP + ΨS = 0 + (-3. 7 bars) = -3. 7 bars

You try it Calculate Ψ of a 0. 15 M solution of of Na. Cl at atmospheric pressure (ΨP = 0) at 25 °C. Note: Na. Cl breaks into 2 pieces so i = 2. ΨS = - (2)(0. 15 mole/liter)(0. 0831 liters bars/mole-K)(298 K) ΨS = - 7. 43 bars Ψ = 0 + (-7. 43 bars) Ψ = -7. 43 bars

You try it again Calculate the solute potential of a 0. 1 M Na. Cl solution at 25 °C. If the Na. CL concentration inside a plant cell is 0. 15 M, which way will the water diffuse if the cell is placed into the 0. 1 M Na. Cl solution? ΨS = - (2)(0. 10 mole/liter)(0. 0831 liters bars/mole-K)(298 K) = - 4. 95 bars (solution) ΨS = - (2)(0. 15 mole/liter)(0. 0831 liters bars/mole-K)(298 K) = - 7. 43 bars (cell) Water will move from solution to cell.

What must Turgor Pressure (ΨP )equal if there is no net diffusion between the solution and the cell? ΨP in cell must equal 2. 49 Goal to make Ψ of cell = Ψ of solution (-4. 95) Ψ = ΨP + ΨS (of cell) Ψ = 2. 49 + (-7. 43) Ψ = -4. 95

Diffusion & Osmosis Lab Read the background material for Lab 4 – Diffusion and Osmosis Procedure 1 – Plasmolysis Procedure 2 – Osmosis & Diffusion on Plant Tissue Procedure 3 – Inquiry
 Phagocytosis vs pinocytosis
Phagocytosis vs pinocytosis Facilitated diffusion vs osmosis
Facilitated diffusion vs osmosis Diffusion osmosis
Diffusion osmosis Diffusion and osmosis
Diffusion and osmosis Water and water and water water
Water and water and water water Adaptation of plants in plains
Adaptation of plants in plains Survive and protect the endangered plants
Survive and protect the endangered plants Osmosis in plants
Osmosis in plants Simple diffusion
Simple diffusion Stimulus diffusion diagram
Stimulus diffusion diagram Water potential definition biology
Water potential definition biology Osmosis water and salt
Osmosis water and salt Vascular vs nonvascular plants
Vascular vs nonvascular plants Non vascular plant reproduction
Non vascular plant reproduction Classify non flowering plants with examples
Classify non flowering plants with examples C3 plant
C3 plant Every living plants and animals must have
Every living plants and animals must have I must become less
I must become less Did madeleine astor survive the titanic
Did madeleine astor survive the titanic