Delineating Toxicity Pathways of Peripheral Neuropathy David Herr


- Slides: 18

Delineating Toxicity Pathways of Peripheral Neuropathy David Herr, Ph. D. Neurotoxicology Division US EPA RTP, NC Mc. Kim Conference on Predictive Toxicology Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division 09/18/2008

What is Neurotoxicity? • Neurotoxicity: “An adverse change in the structure or function of the central and/or peripheral nervous system following exposure to a chemical, physical, or biological agent” 1 • Adverse Effect: “Alterations from baseline or normal conditions that diminish an organism’s ability to survive, reproduce, or adapt to the environment” 1 1 Guidelines for Neurotoxicity Risk Assessment Federal Register, 63(93): 26926 -26954, 1998 http: //cfpub. epa. gov/ncea/raf/recordisplay. cfm? deid=12479 Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division 1

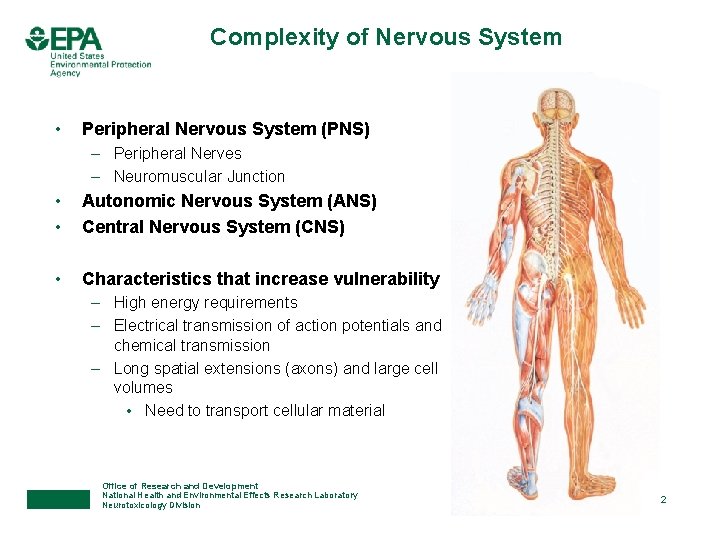
Complexity of Nervous System • Peripheral Nervous System (PNS) – Peripheral Nerves – Neuromuscular Junction • Autonomic Nervous System (ANS) Central Nervous System (CNS) • Characteristics that increase vulnerability • – High energy requirements – Electrical transmission of action potentials and chemical transmission – Long spatial extensions (axons) and large cell volumes • Need to transport cellular material Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division 2

Signs of Peripheral/Central Neuropathies • Biochemical – – • • – – Neurofilament accumulations Changes in axonal transport Altered myelin Altered ion gradients Physiological – Decreased amplitudes of nerve action potentials – Decreased nerve conduction velocity – Denervation potentials in muscles – Alterations in somatosensory evoked potentials Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division Behavioral • Parasthesias Increased reaction time Vibrotactile abnormalities Paralysis Pathological – Neuronal / Axonal degeneration – Changes in myelin 3

NRC 21 st Centrury Toxicology Testing Paradigm QSAR Reversibility Systems Biology: Define normal function and its bounds, where further insult compromises function and leads to toxicity Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division NRC, 2007 4

Patterns of Neurotoxic Injury • Neuronopathy: Death of entire neuron • Astrocyte proliferation • Axonopathy: Axon degenerates • Neuronal chromatolysis • Nissl substance and nucleus to periphery • Myelinopathy: Injury to Schwann or Oligodendrocytes Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division Adapted From: Anthony et al. , Casarett & Doull, 2001 5

Types of Toxicity – Neuronopathies • Primary “Neuronal” Site of Action – Metabolic Inhibitors: 6 -Amino-nicotinamide, arsenic – Inhibit DNA/Protein Synthesis: cloramphenicol, doxorubicin – Protein Binding: thallium, methyl mercury (? ) – Excitotoxicity: B-N-Methylamino-L-alanine (BMAA) • Multiple chemical classes, Modes of action(s) – No single event leads to neuronal death – Overwhelm repair process = f: (dose, duration, persistence, repair) – Can model NMDA receptors, metabolic inhibitors (? ), propensity for protein binding (? ), etc… – What is critical event to produce neuropathy? Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division 6

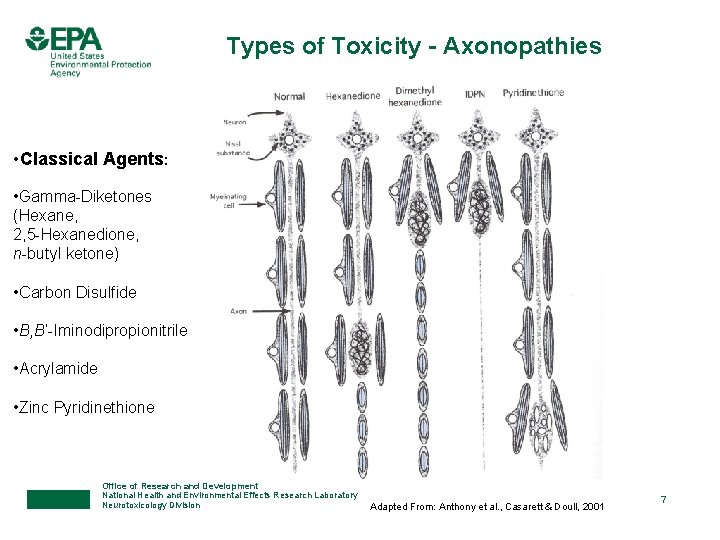
Types of Toxicity - Axonopathies • Classical Agents: • Gamma-Diketones (Hexane, 2, 5 -Hexanedione, n-butyl ketone) • Carbon Disulfide • B, B’-Iminodipropionitrile • Acrylamide • Zinc Pyridinethione Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division Adapted From: Anthony et al. , Casarett & Doull, 2001 7

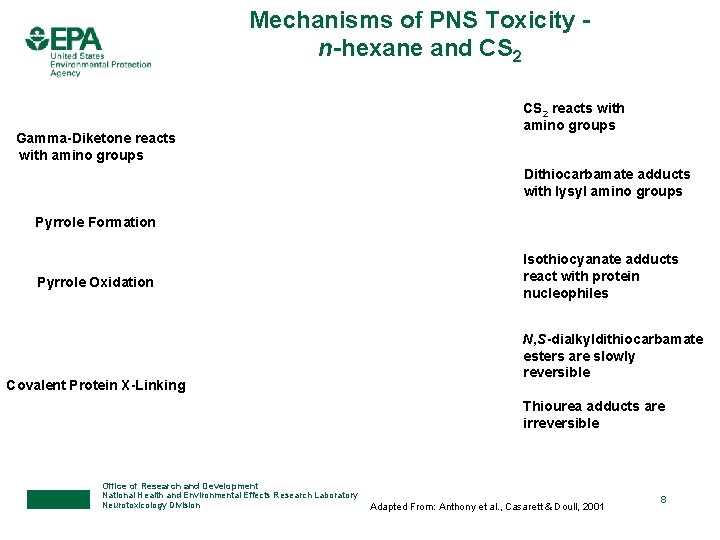
Mechanisms of PNS Toxicity n-hexane and CS 2 Gamma-Diketone reacts with amino groups CS 2 reacts with amino groups Dithiocarbamate adducts with lysyl amino groups Pyrrole Formation Pyrrole Oxidation Covalent Protein X-Linking Isothiocyanate adducts react with protein nucleophiles N, S-dialkyldithiocarbamate esters are slowly reversible Thiourea adducts are irreversible Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division Adapted From: Anthony et al. , Casarett & Doull, 2001 8

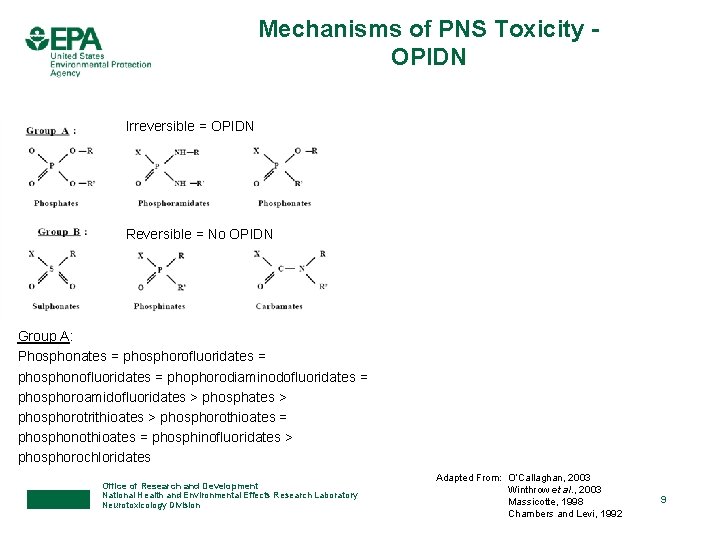
Mechanisms of PNS Toxicity OPIDN Irreversible = OPIDN Reversible = No OPIDN Group A: Phosphonates = phosphorofluoridates = phosphonofluoridates = phophorodiaminodofluoridates = phosphoroamidofluoridates > phosphorotrithioates > phosphorothioates = phosphonothioates = phosphinofluoridates > phosphorochloridates Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division Adapted From: O’Callaghan, 2003 Winthrow et al. , 2003 Massicotte, 1998 Chambers and Levi, 1992 9

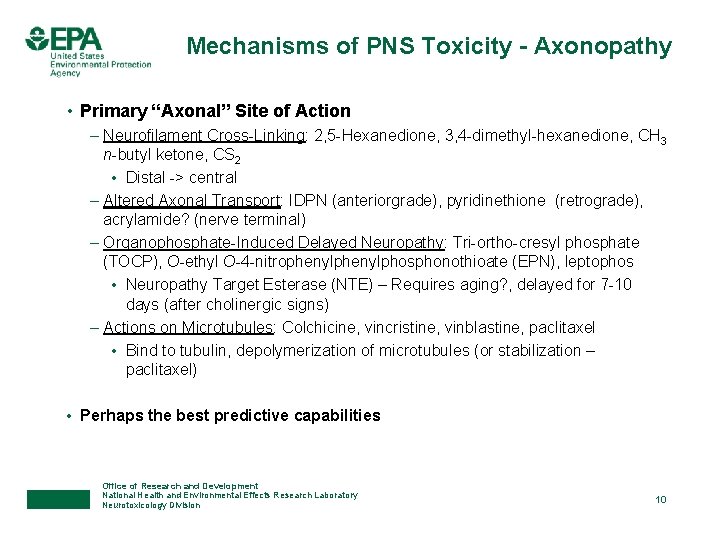
Mechanisms of PNS Toxicity - Axonopathy • Primary “Axonal” Site of Action – Neurofilament Cross-Linking: 2, 5 -Hexanedione, 3, 4 -dimethyl-hexanedione, CH 3 n-butyl ketone, CS 2 • Distal -> central – Altered Axonal Transport: IDPN (anteriorgrade), pyridinethione (retrograde), acrylamide? (nerve terminal) – Organophosphate-Induced Delayed Neuropathy: Tri-ortho-cresyl phosphate (TOCP), O-ethyl O-4 -nitrophenylphosphonothioate (EPN), leptophos • Neuropathy Target Esterase (NTE) – Requires aging? , delayed for 7 -10 days (after cholinergic signs) – Actions on Microtubules: Colchicine, vincristine, vinblastine, paclitaxel • Bind to tubulin, depolymerization of microtubules (or stabilization – paclitaxel) • Perhaps the best predictive capabilities Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division 10

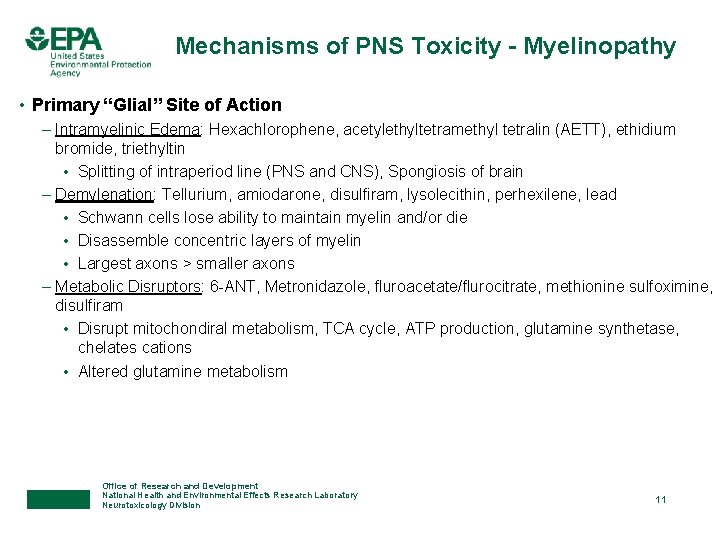
Mechanisms of PNS Toxicity - Myelinopathy • Primary “Glial” Site of Action – Intramyelinic Edema: Hexachlorophene, acetylethyltetramethyl tetralin (AETT), ethidium bromide, triethyltin • Splitting of intraperiod line (PNS and CNS), Spongiosis of brain – Demylenation: Tellurium, amiodarone, disulfiram, lysolecithin, perhexilene, lead • Schwann cells lose ability to maintain myelin and/or die • Disassemble concentric layers of myelin • Largest axons > smaller axons – Metabolic Disruptors: 6 -ANT, Metronidazole, fluroacetate/flurocitrate, methionine sulfoximine, disulfiram • Disrupt mitochondiral metabolism, TCA cycle, ATP production, glutamine synthetase, chelates cations • Altered glutamine metabolism Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division 11

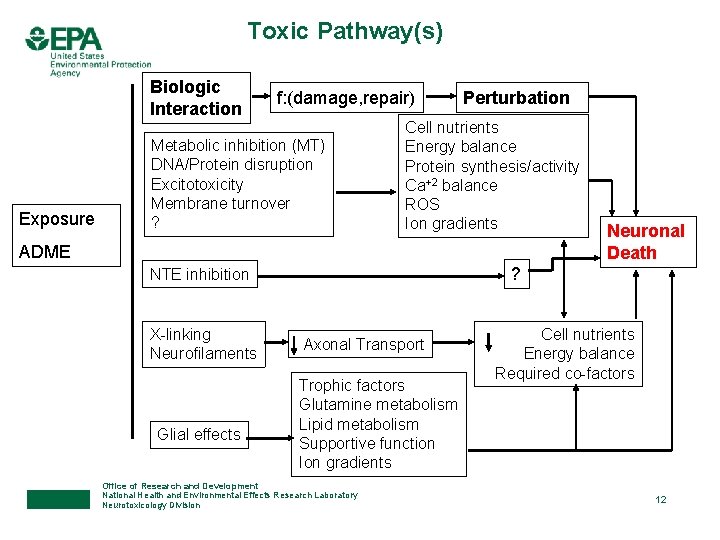
Toxic Pathway(s) Biologic Interaction Exposure f: (damage, repair) Metabolic inhibition (MT) DNA/Protein disruption Excitotoxicity Membrane turnover ? Perturbation Cell nutrients Energy balance Protein synthesis/activity Ca+2 balance ROS Ion gradients ADME ? NTE inhibition X-linking Neurofilaments Glial effects Neuronal Death Axonal Transport Trophic factors Glutamine metabolism Lipid metabolism Supportive function Ion gradients Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division Cell nutrients Energy balance Required co-factors 12

Challenges for the Future – Systems Biology and QSAR • System-level understanding of biological processes – Initiating events and subsequent downstream critical events • Model Components: – Structure of the system (gene regulatory systems, biochemical networks, and physical structures) – Dynamics (changes over time) of the system, both quantitatively and qualitatively • Theory/model needs predictive capability – Biological controls of the system • Appropriate tools to design the system Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division 13

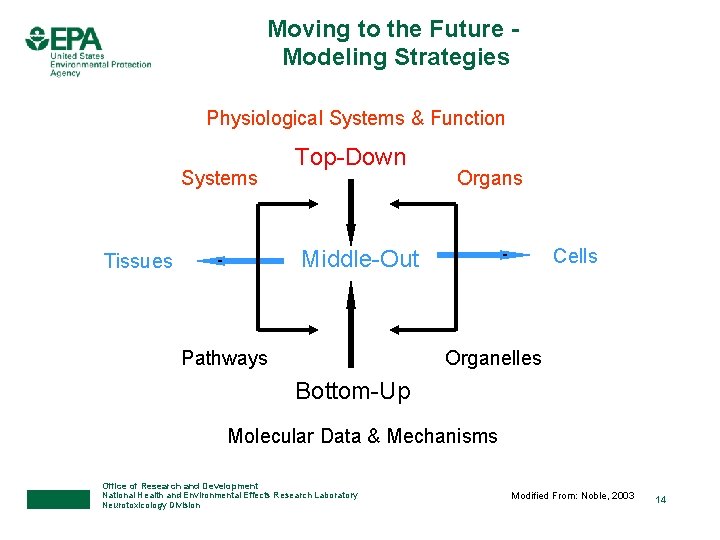
Moving to the Future Modeling Strategies Physiological Systems & Function Systems Top-Down Organs Cells Middle-Out Tissues Pathways Organelles Bottom-Up Molecular Data & Mechanisms Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division Modified From: Noble, 2003 14

Thanks for your time and attention! Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division 15

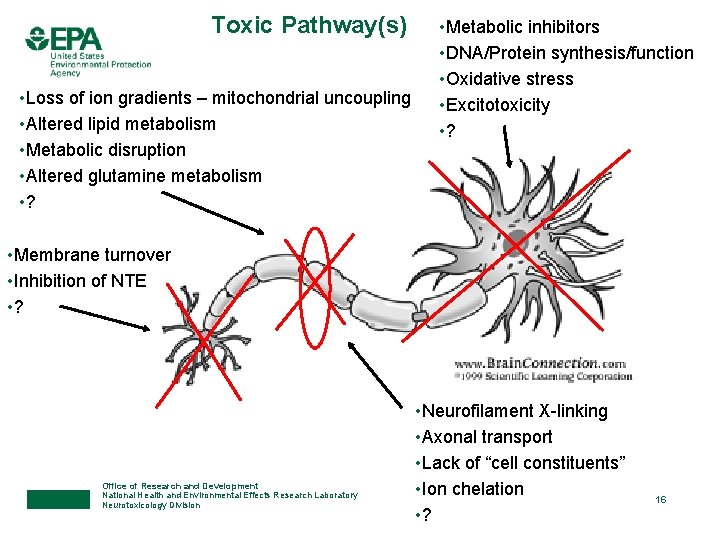
Toxic Pathway(s) • Loss of ion gradients – mitochondrial uncoupling • Altered lipid metabolism • Metabolic disruption • Altered glutamine metabolism • ? • Metabolic inhibitors • DNA/Protein synthesis/function • Oxidative stress • Excitotoxicity • ? • Membrane turnover • Inhibition of NTE • ? Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division • Neurofilament X-linking • Axonal transport • Lack of “cell constituents” • Ion chelation • ? 16

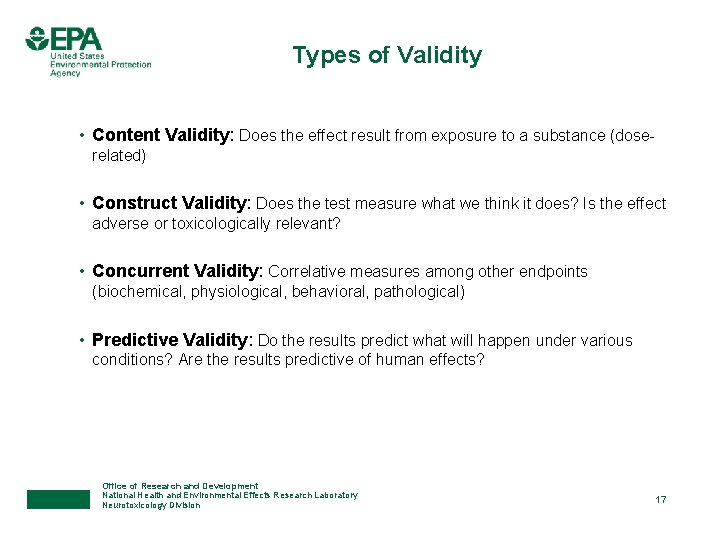
Types of Validity • Content Validity: Does the effect result from exposure to a substance (doserelated) • Construct Validity: Does the test measure what we think it does? Is the effect adverse or toxicologically relevant? • Concurrent Validity: Correlative measures among other endpoints (biochemical, physiological, behavioral, pathological) • Predictive Validity: Do the results predict what will happen under various conditions? Are the results predictive of human effects? Office of Research and Development National Health and Environmental Effects Research Laboratory Neurotoxicology Division 17
 Lied herr du bist mein leben
Lied herr du bist mein leben Delineating an argument
Delineating an argument Respiratory failure type 1
Respiratory failure type 1 Ideopathic peripheral neuropathy
Ideopathic peripheral neuropathy Diabetes neuropathy
Diabetes neuropathy Diabetic neuropathy schaumburg
Diabetic neuropathy schaumburg Extensor retinaculum
Extensor retinaculum Albuminocytologic dissociation
Albuminocytologic dissociation Neuropathy
Neuropathy Norma compression stockings
Norma compression stockings Description of pain
Description of pain Diabetic autonomic neuropathy
Diabetic autonomic neuropathy Giant cell arteritis
Giant cell arteritis Large fibre neuropathy
Large fibre neuropathy Neuropathy test
Neuropathy test Chiropractic marketing flyers
Chiropractic marketing flyers Herr dr. medic. (im tem.) adrian balint
Herr dr. medic. (im tem.) adrian balint Why is chapter 6 called the overpaid maid
Why is chapter 6 called the overpaid maid Julien herr
Julien herr