Defining the Atom Pearson Prentice Hall Physical Science






























- Slides: 42

Defining the Atom > Pearson Prentice Hall Physical Science: Concepts in Action Chapter 4 Slide 1 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > Slide 2 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > Atom • An atom is the smallest particle of an element • Element: an element is a substance that cannot be broken down in simpler substances. It is a single type of atom ATOMS NOT ADAMS This Photo by Unknown Author is licensed under CC BY-NC-ND © Copyright Pearson Prentice Hall Slide 3 of 18 End Show

4. 1 Defining the Atom > Sizing up the Atom Despite their small size, individual atoms are observable with instruments such as scanning tunneling microscopes. Slide 4 of 18 © Copyright Pearson Prentice Hall End Show

4. 2 Defining the Atom > Subatomic Particles Atoms are made up of three subatomic particles. What are three kinds of subatomic particles? Slide 5 of 18 © Copyright Pearson Prentice Hall End Show

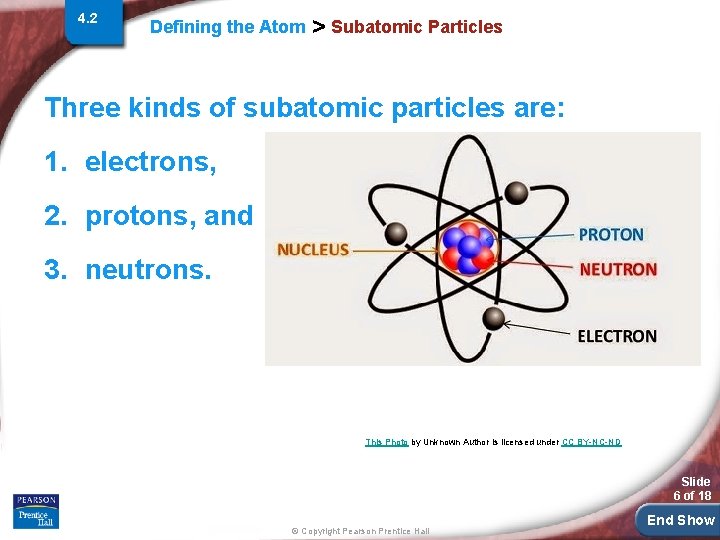
4. 2 Defining the Atom > Subatomic Particles Three kinds of subatomic particles are: 1. electrons, 2. protons, and 3. neutrons. This Photo by Unknown Author is licensed under CC BY-NC-ND Slide 6 of 18 © Copyright Pearson Prentice Hall End Show

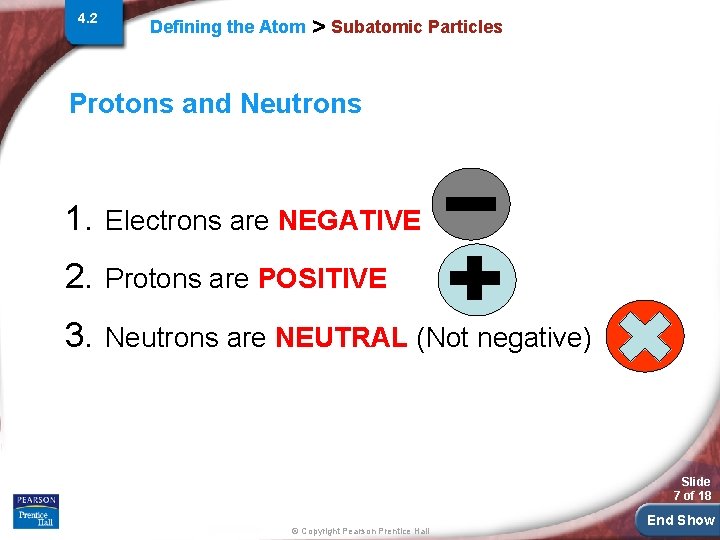
4. 2 Defining the Atom > Subatomic Particles Protons and Neutrons 1. Electrons are NEGATIVE 2. Protons are POSITIVE 3. Neutrons are NEUTRAL (Not negative) Slide 7 of 18 © Copyright Pearson Prentice Hall End Show

4. 2 Defining the Atom > Subatomic Particles Protons and Neutrons 1. Electrons are NEGATIVE a) They are very small and we generally say they have no mass b) They are found outside the nucleus (center) of the atom Slide 8 of 18 © Copyright Pearson Prentice Hall End Show

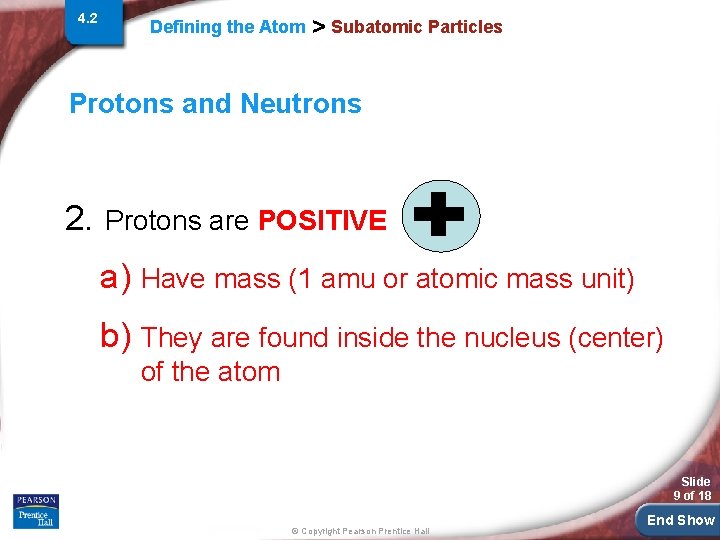
4. 2 Defining the Atom > Subatomic Particles Protons and Neutrons 2. Protons are POSITIVE a) Have mass (1 amu or atomic mass unit) b) They are found inside the nucleus (center) of the atom Slide 9 of 18 © Copyright Pearson Prentice Hall End Show

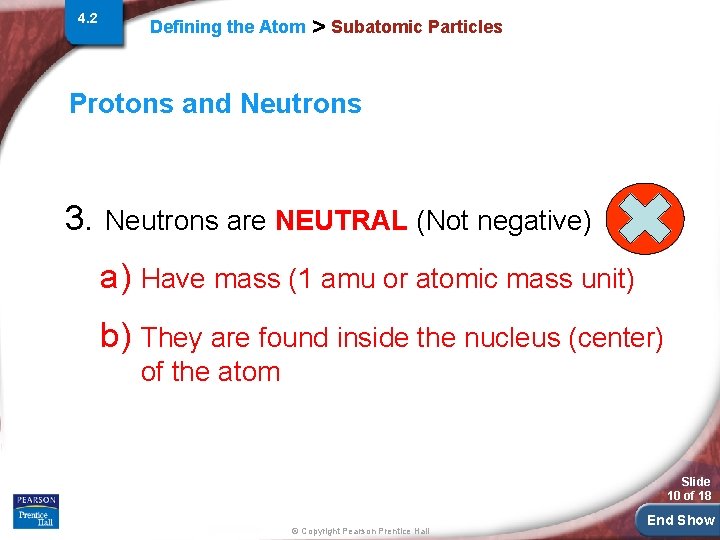
4. 2 Defining the Atom > Subatomic Particles Protons and Neutrons 3. Neutrons are NEUTRAL (Not negative) a) Have mass (1 amu or atomic mass unit) b) They are found inside the nucleus (center) of the atom Slide 10 of 18 © Copyright Pearson Prentice Hall End Show

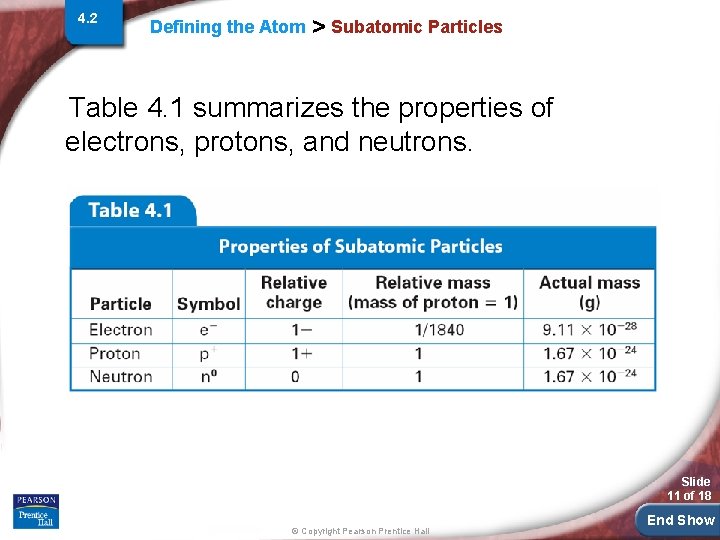
4. 2 Defining the Atom > Subatomic Particles Table 4. 1 summarizes the properties of electrons, protons, and neutrons. Slide 11 of 18 © Copyright Pearson Prentice Hall End Show

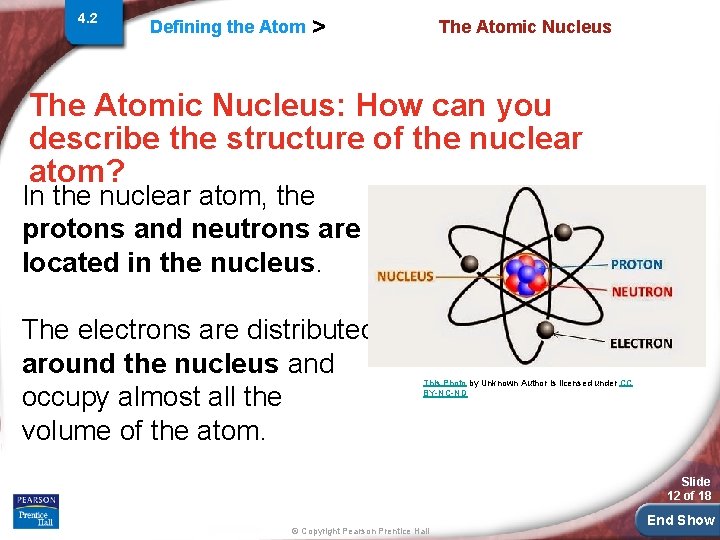
4. 2 Defining the Atom > The Atomic Nucleus: How can you describe the structure of the nuclear atom? In the nuclear atom, the protons and neutrons are located in the nucleus. The electrons are distributed around the nucleus and occupy almost all the volume of the atom. This Photo by Unknown Author is licensed under CC BY-NC-ND Slide 12 of 18 © Copyright Pearson Prentice Hall End Show

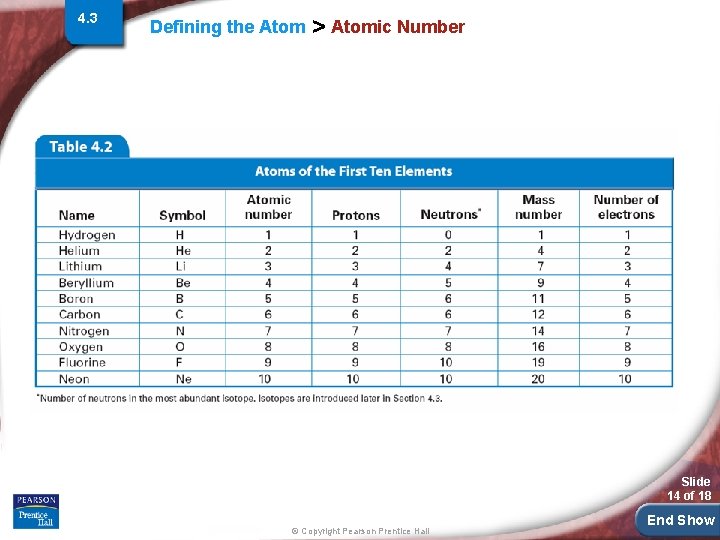
4. 3 Defining the Atom > Atomic Number Elements are different because they contain different numbers of protons. The atomic number of an element is the number of protons in the nucleus of an atom of that element. Slide 13 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Atomic Number Slide 14 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Mass Number How do you find the number of neutrons in an atom? Slide 15 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Mass Number The total number of protons and neutrons in an atom is called the mass number. • The number of neutrons in an atom is the difference between the mass number and atomic number. Slide 16 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Mass Number Au is the chemical symbol for gold. Slide 17 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Isotopes How do isotopes of an element differ? Slide 18 of 18 © Copyright Pearson Prentice Hall End Show

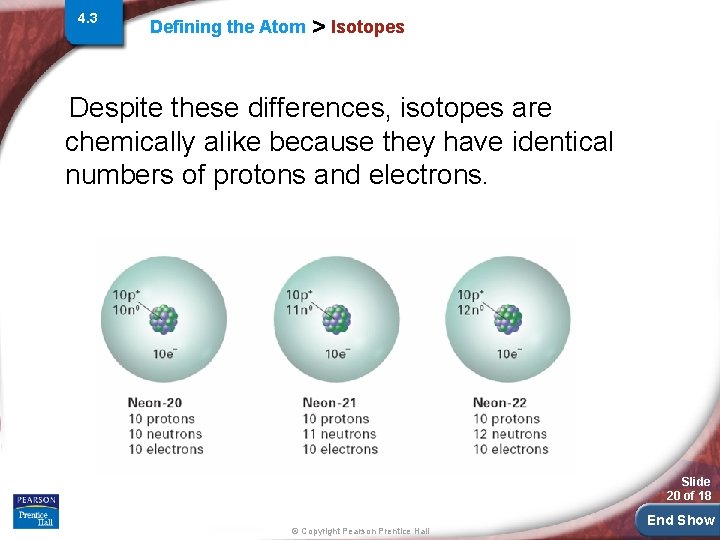
4. 3 Defining the Atom > Isotopes are atoms that have the same number of protons but different numbers of neutrons. • Because isotopes of an element have different numbers of neutrons, they also have different mass numbers. Slide 19 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Isotopes Despite these differences, isotopes are chemically alike because they have identical numbers of protons and electrons. Slide 20 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Atomic Mass How do you calculate the atomic mass of an element? Slide 21 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Atomic Mass It is useful to to compare the relative masses of atoms to a standard reference isotope. Carbon 12 is the standard reference isotope. Cabon-12 has a mass of exactly 12 atomic mass units. An atomic mass unit (amu) is defined as one twelfth of the mass of a carbon-12 atom. Slide 22 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Atomic Mass The atomic mass of an element is a weighted average mass of the atoms in a naturally occurring sample of the element. A weighted average mass reflects both the mass and the relative abundance of the isotopes as they occur in nature. Slide 23 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Atomic Mass To calculate the atomic mass of an element, multiply the mass of each isotope by its natural abundance, expressed as a decimal, and then add the products. Slide 24 of 18 © Copyright Pearson Prentice Hall End Show

4. 3 Defining the Atom > Atomic Mass For example, carbon has two stable isotopes: • Carbon-12, which has a natural abundance of 98. 89%, and • Carbon-13, which has a natural abundance of 1. 11%. Slide 25 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > 4. 2 Slide 26 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > Mendeleev’s Organization Mendeleev arranged the elements in order of increasing mass Elements with similar properties were in the same column by using this arrangement Definition: a periodic table is an arrangement of elements in columns based on a set of properties that repeat from row to row Slide 27 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > Evidence for the Mendeleev Table Mendeleev used his organization of the table to predict where undiscovered elements would fit He deliberately left spaces or gaps in the table for these elements As new elements were discovered, Mendeleev’s prediction proved to be correct The close match between the predictions and the actual properties of new elements showed how useful his periodic table could be Slide 28 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom Objectives: > 5. 2 The Modern Periodic Table 1. Describe how the modern periodic table is organized 2. Explain what atomic mass of an element depends on 3. Compare the categories that are used to classify elements on the periodic table 4. Distinguish how properties vary across a period in the periodic table Slide 29 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > Organization of the Modern Periodic Table In the modern periodic table, elements are arranged by increasing atomic number Atomic number is the number of protons Each ROW of the table is a PERIOD Each COLUMN is a GROUP or FAMILY Properties of elements repeat predictably when atomic numbers are used to arrange elements in groups Definition: periodic law is the predictable End Show pattern of repeating properties in the periodic Slide 30 of 18 © Copyright Pearson Prentice Hall

Atomic Mass Defining the Atom > Atomic mass is a value that depends in the distribution of an element’s isotopes in nature and the masses of those isotopes Definition: an atomic mass unit (amu) is one twelfth the mass of a carbon-12 atom Mass is given in grams Scientists assigned 12 atomic mass units (amu) or 12 g to the carbon-12 atom which has 6 protons and 6 neutrons Each proton and each neutron weighs one gram Slide 31 of 18 Electron mass is small and is ignored under this End Show © Copyright Pearson Prentice Hall

Defining the Atom > Definition: an isotope of an element has a different number of neutrons in its nucleus Since mass is given by protons plus neutrons, isotopes of an element have different masses It is still the same the element, however, because the number of PROTONS remains unchanged The number of protons determines which element you have Slide 32 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > Most elements exist as a mixture of two or more isotopes http: //yteach. co. uk/page. php/resources/view_ all? id=atom_atomic_number_mass_nuclei_sy mbol_isotope_electron_proton_t_page_8&fro m=search Atomic mass is determined by the weighted average of all the isotopes The weighted average takes into account how often the isotope occurs by using percentages Slide 33 of 18 End Show The more often an isotope occurs, the more it © Copyright Pearson Prentice Hall

Defining the Atom > 3 Categories to Classify Elements are classified as metals, nonmetals and metalloids Metalloids are also called semiconductors Metals are good conductors, ductile (can be drawn into wires), malleable, shiny, & solid (with the exception of mercury) Transition metals (flat part of the table) tend to form compounds with color Nonmetals are poor conductors, have low boiling points & tend to be gases Slide 34 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > How Properties Vary Across the Table Periods are the rows Across a period from left to right, the elements become less metallic and more nonmetallic Slide 35 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > 5. 3 Representative Groups Objectives: 1. Explain why elements in a group have similar properties 2. Describe some properties of the Group A (representative) elements Slide 36 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > Similar Properties in a Group Definition: a valence electron is an electron that is in the highest occupied energy level of an atom Valence electrons are the electrons in the “outer shell” Elements in a group have similar properties because they have the same number of valence electrons Elements seek to have complete outer shells with 8 electrons Therefore, the number of valence © Copyright Pearson Prentice Hall Slide 37 of 18 End Show

Group. Defining A the Atom > Group 1 A are the alkali metals The reactivity of alkali metals increases from the top of Group 1 A to the bottom Group 1 A has one valence electron (charge is +1) Group 2 a are the alkaline earth metals Differences in reactivity among the alkaline earth metals are shown by the ways they react with water: calcium, strontium & barium react easily These elements have 2 valence electrons © Copyright Pearson Prentice Hall Slide 38 of 18 End Show

Group 3 A Defining is thetheboron Atom > family Aluminum is in this family & it is the most abundant METAL in earth’s crust Group 3 A has 3 valence electrons (charge is +3) Group 4 A is the carbon family The carbon family has 4 valence electrons Except for water, most of the compounds in your body contain carbon These elements are reactive and will either give up their 4 valence electrons or share 4 valence End Show electrons with other elements (charge is either Slide 39 of 18 © Copyright Pearson Prentice Hall

The nitrogen family is Group 5 A Defining the Atom > Group 5 A has 5 valence electrons which it can give up (charge +5) Group 5 A can also take in 3 additional electrons from other elements which make a charge of -3 This group is a mixture one metal plus nonmetals & metalloids The oxygen family is Group 6 A It has both nonmetals & metalloids Oxygen is most abundant ELEMENT in the earth’s crust Slide 40 of 18 These elements have 6 valence electrons © Copyright Pearson Prentice Hall End Show

Defining the Atom > Group 7 A are the halogens “halo” means salt & “gen” comes from genesis which means beginning or formation Halogens are salt formers They readily react with metals Their 7 valence electrons means they seek one additional electron, giving their family a charge of -1 Slide 41 of 18 © Copyright Pearson Prentice Hall End Show

Defining the Atom > Group 8 A are the noble gases The nobles are colorless, odorless and unreactive because they have 8 valence electrons in their outer shell Since their outer shell is complete their charge is 0 The nobles are also called the zero elements Slide 42 of 18 © Copyright Pearson Prentice Hall End Show