Deduction of Fundamental Laws for Heat Exchangers P



- Slides: 36

Deduction of Fundamental Laws for Heat Exchangers P M V Subbarao Professor Mechanical Engineering Department I I T Delhi Modification of Basic Laws for Design of General Templates for HXs? !? !? !

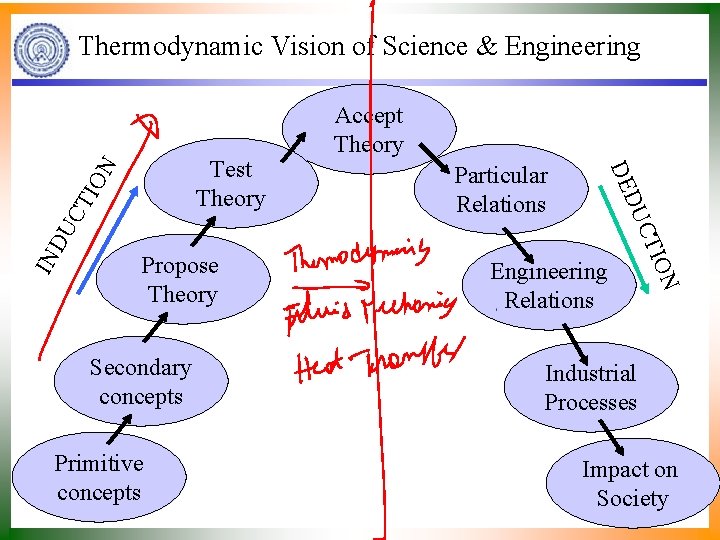
Thermodynamic Vision of Science & Engineering N IO CT DU IN Primitive concepts Engineering Relations ION Secondary concepts CT Propose Theory Particular Relations DU DE Test Theory Accept Theory Industrial Processes Impact on Society

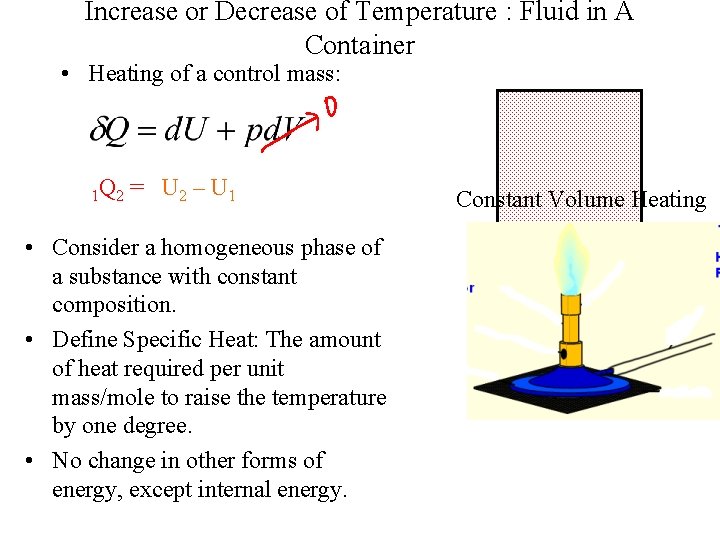
Increase or Decrease of Temperature : Fluid in A Container • Heating of a control mass: 1 Q 2 = U 2 – U 1 • Consider a homogeneous phase of a substance with constant composition. • Define Specific Heat: The amount of heat required per unit mass/mole to raise the temperature by one degree. • No change in other forms of energy, except internal energy. Constant Volume Heating

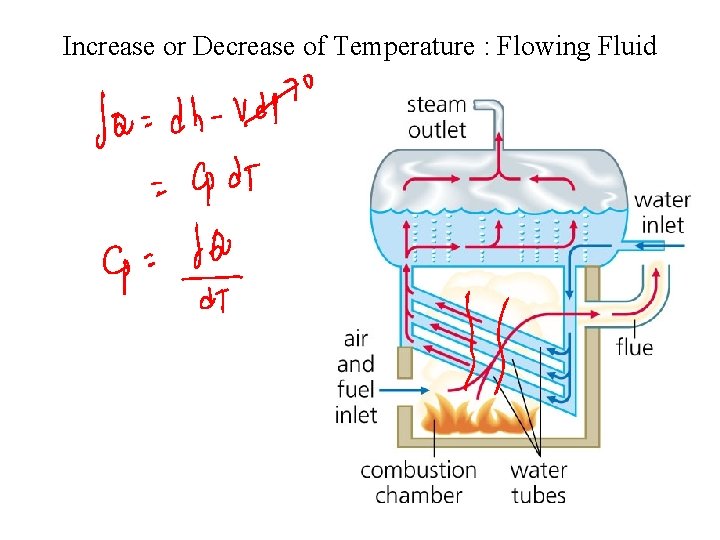
Increase or Decrease of Temperature : Flowing Fluid

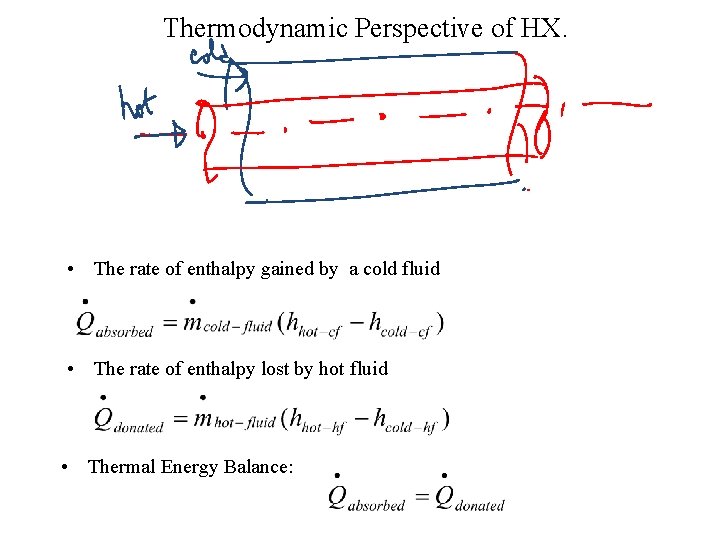
Thermodynamic Perspective of HX. • The rate of enthalpy gained by a cold fluid • The rate of enthalpy lost by hot fluid • Thermal Energy Balance:

Heat Transfer Perspective of HX. • • Estimation and Creation of primary driving force. To the hot fluid loose thermal energy? To help cold fluid gain thermal energy? Provision of thermal infrastructure to satisfy law of conservation of energy. • How to model this mutual interaction using principles of Heat Transfer ? Understanding of precise role of thermodynamic Parameters…

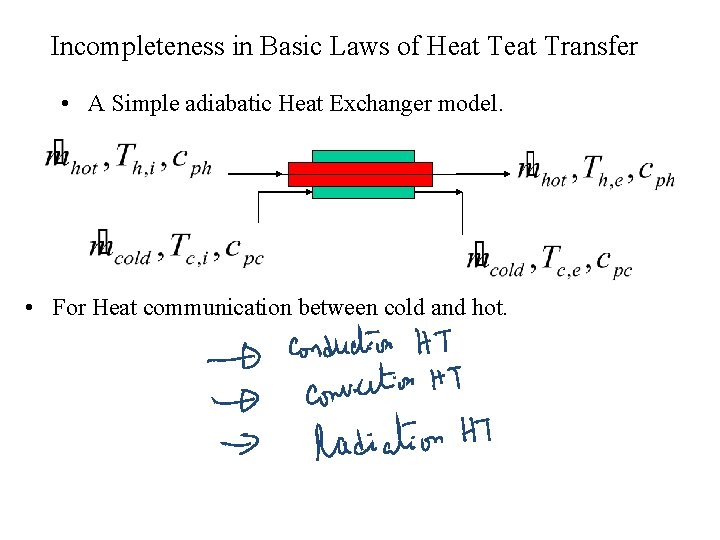
Incompleteness in Basic Laws of Heat Transfer • A Simple adiabatic Heat Exchanger model. • For Heat communication between cold and hot.

Fourier’s law of heat conduction A Constitutive Relation This is called as Fourier Law of Conduction Global heat transfer rate:

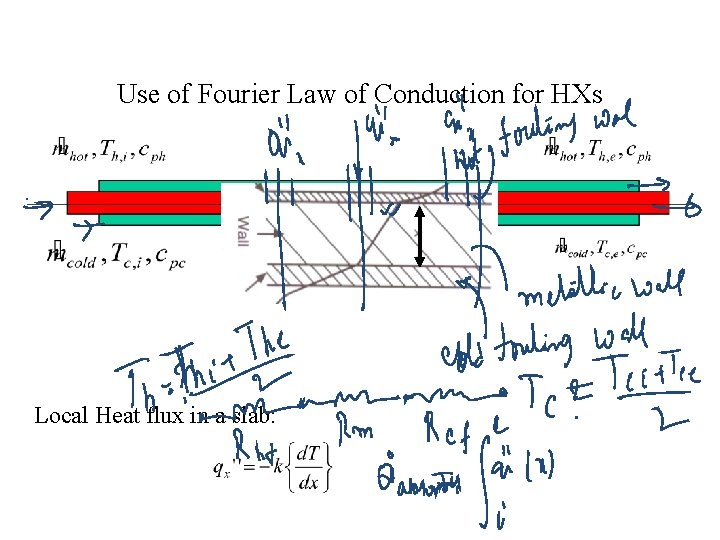
Use of Fourier Law of Conduction for HXs Local Heat flux in a slab:

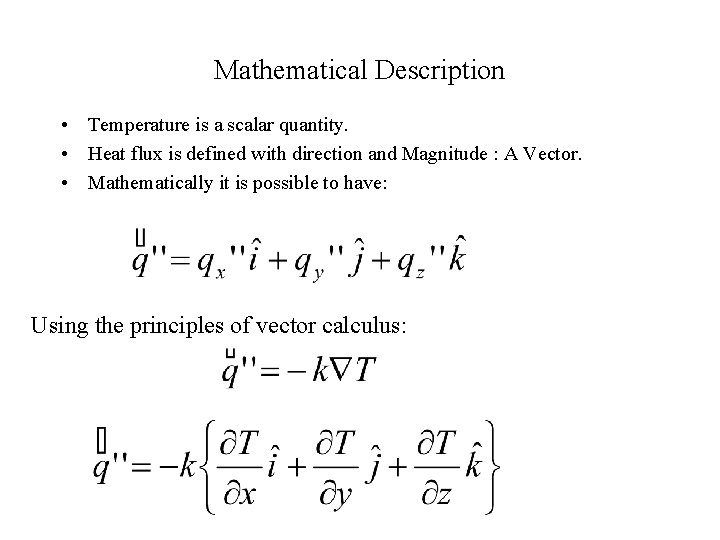
Mathematical Description • Temperature is a scalar quantity. • Heat flux is defined with direction and Magnitude : A Vector. • Mathematically it is possible to have: Using the principles of vector calculus:

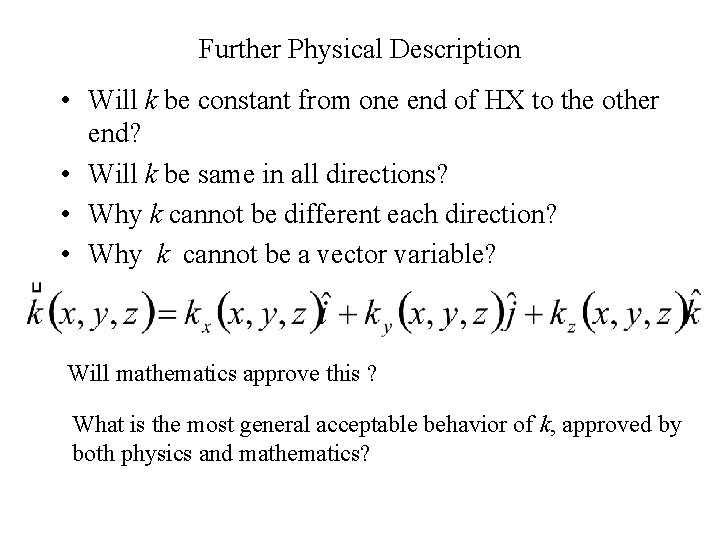
Further Physical Description • Will k be constant from one end of HX to the other end? • Will k be same in all directions? • Why k cannot be different each direction? • Why k cannot be a vector variable? Will mathematics approve this ? What is the most general acceptable behavior of k, approved by both physics and mathematics?

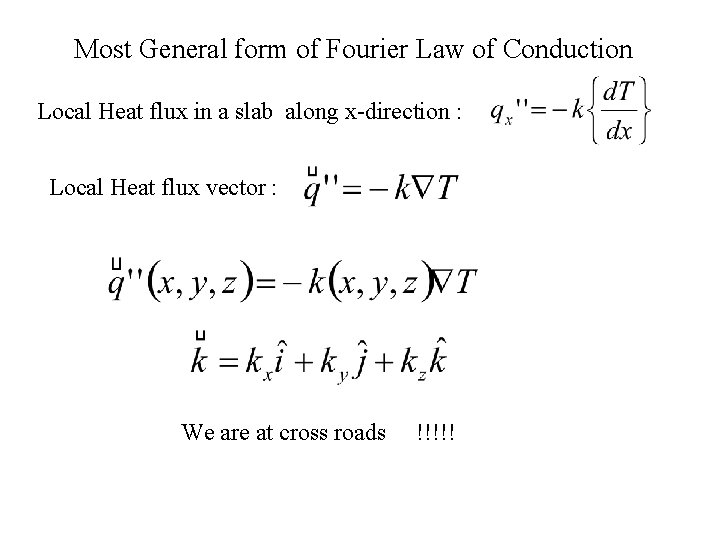
Most General form of Fourier Law of Conduction Local Heat flux in a slab along x-direction : Local Heat flux vector : We are at cross roads !!!!!

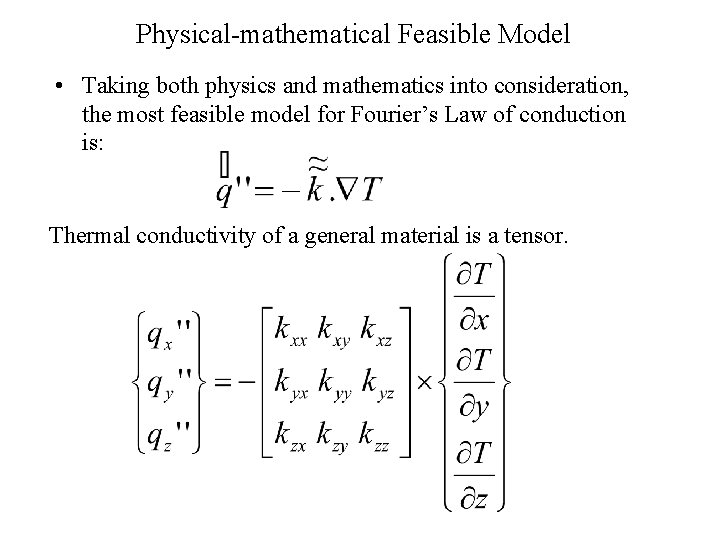
Physical-mathematical Feasible Model • Taking both physics and mathematics into consideration, the most feasible model for Fourier’s Law of conduction is: Thermal conductivity of a general material is a tensor.

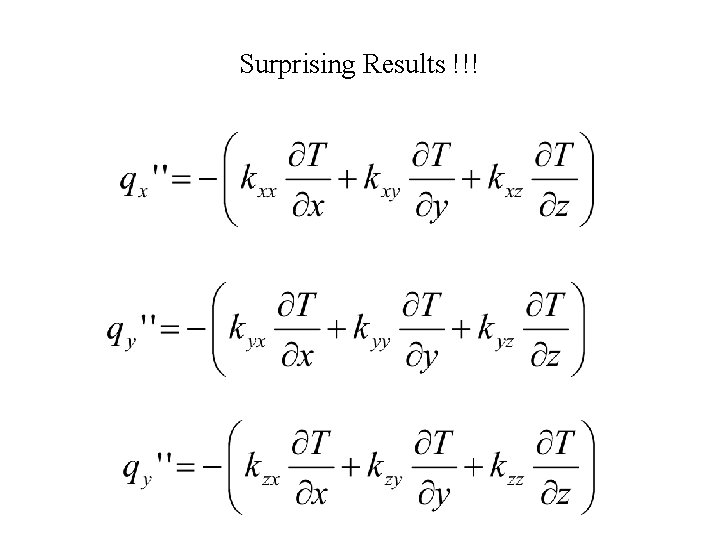
Surprising Results !!!

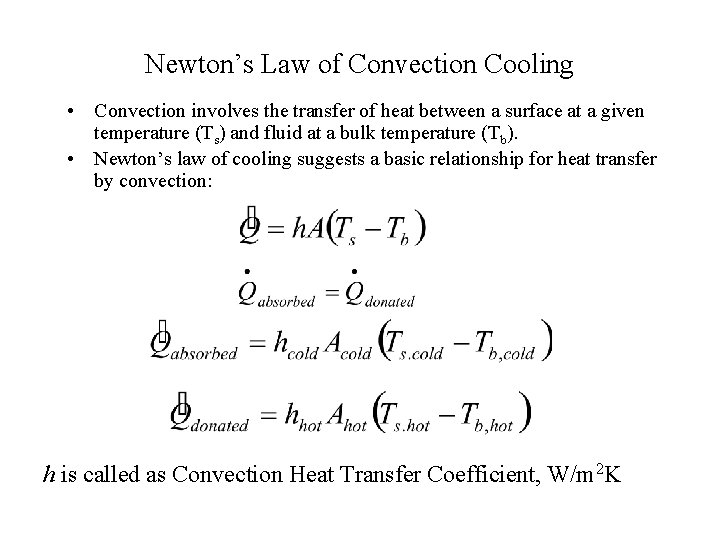
Newton’s Law of Convection Cooling • Convection involves the transfer of heat between a surface at a given temperature (Ts) and fluid at a bulk temperature (Tb). • Newton’s law of cooling suggests a basic relationship for heat transfer by convection: h is called as Convection Heat Transfer Coefficient, W/m 2 K

Realization of Newton’s Law Cooling • A general heat transfer surface may not be isothermal !? ! • Fluid temperature will vary from inlet to exit !? !? ! • The local velocity of flow will also vary from inlet to exit ? !? ! • How to use Newton’s Law in a Real life?

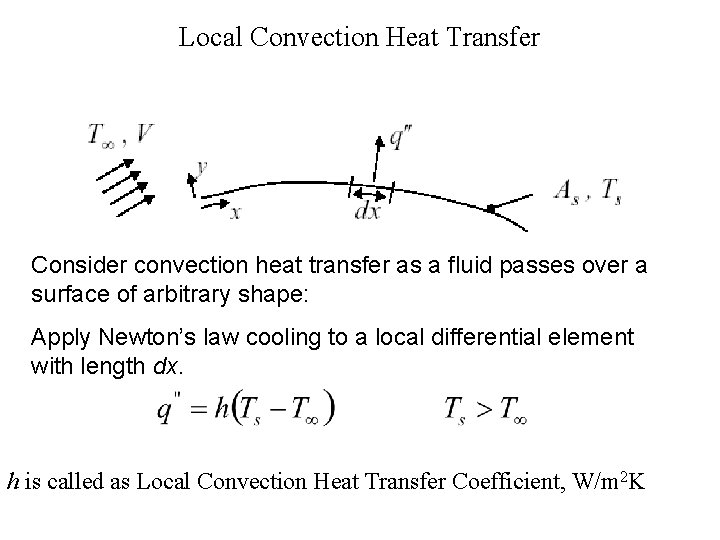
Local Convection Heat Transfer Consider convection heat transfer as a fluid passes over a surface of arbitrary shape: Apply Newton’s law cooling to a local differential element with length dx. h is called as Local Convection Heat Transfer Coefficient, W/m 2 K

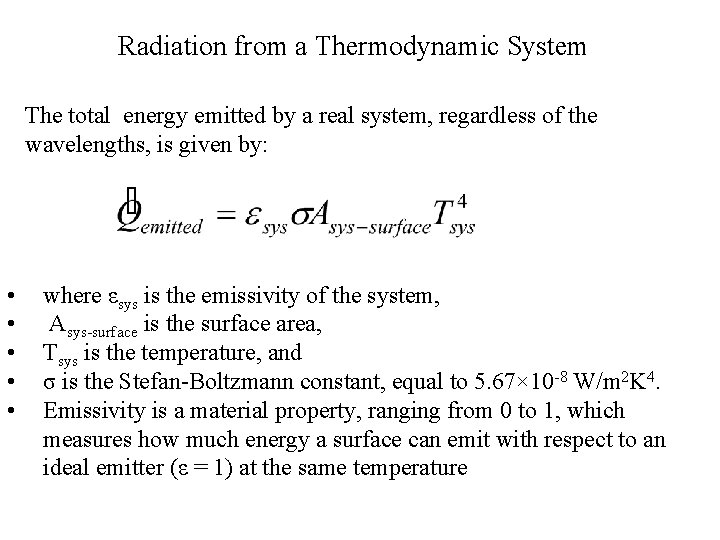
Radiation from a Thermodynamic System The total energy emitted by a real system, regardless of the wavelengths, is given by: • • • where εsys is the emissivity of the system, Asys-surface is the surface area, Tsys is the temperature, and σ is the Stefan-Boltzmann constant, equal to 5. 67× 10 -8 W/m 2 K 4. Emissivity is a material property, ranging from 0 to 1, which measures how much energy a surface can emit with respect to an ideal emitter (ε = 1) at the same temperature

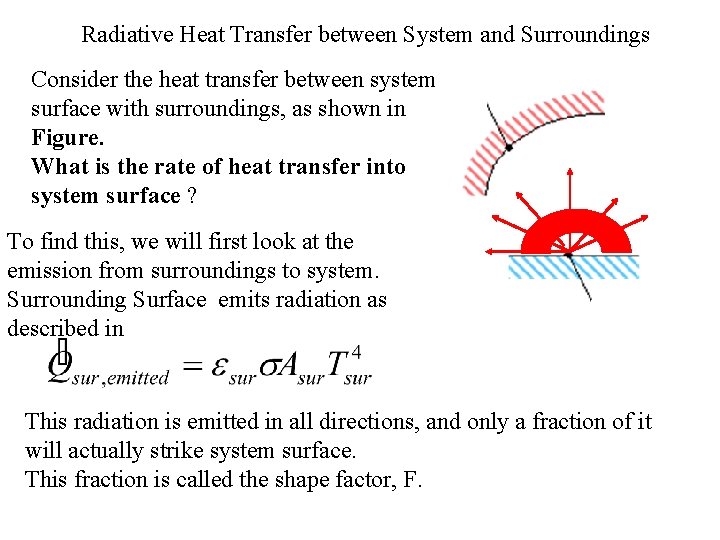
Radiative Heat Transfer between System and Surroundings Consider the heat transfer between system surface with surroundings, as shown in Figure. What is the rate of heat transfer into system surface ? To find this, we will first look at the emission from surroundings to system. Surrounding Surface emits radiation as described in This radiation is emitted in all directions, and only a fraction of it will actually strike system surface. This fraction is called the shape factor, F.

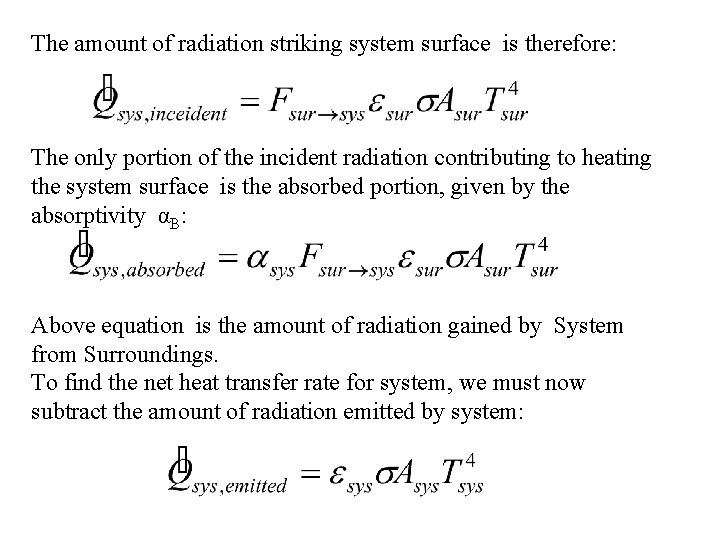
The amount of radiation striking system surface is therefore: The only portion of the incident radiation contributing to heating the system surface is the absorbed portion, given by the absorptivity αB: Above equation is the amount of radiation gained by System from Surroundings. To find the net heat transfer rate for system, we must now subtract the amount of radiation emitted by system:

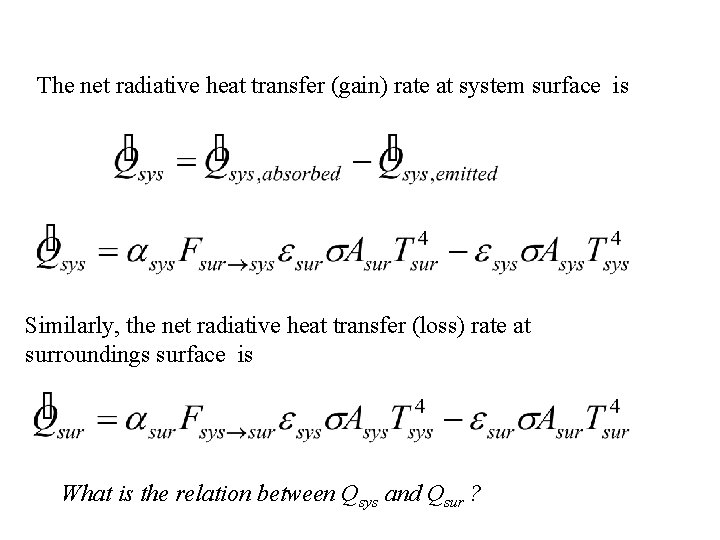
The net radiative heat transfer (gain) rate at system surface is Similarly, the net radiative heat transfer (loss) rate at surroundings surface is What is the relation between Qsys and Qsur ?

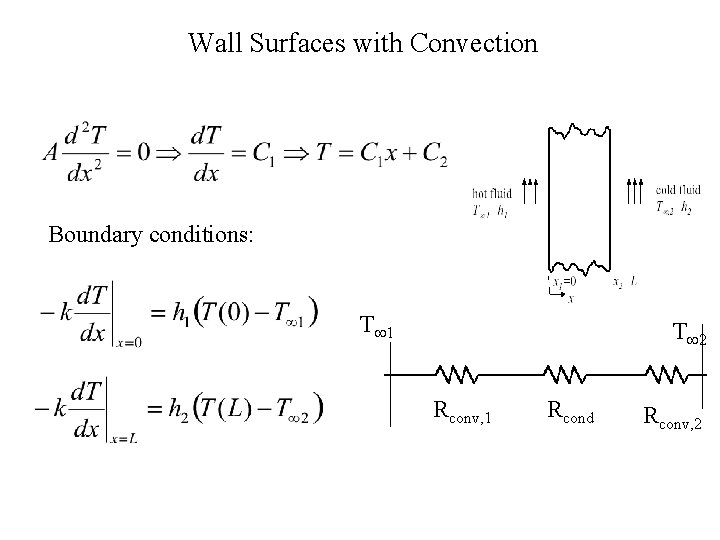
Wall Surfaces with Convection Boundary conditions: T 1 T 2 Rconv, 1 Rcond Rconv, 2

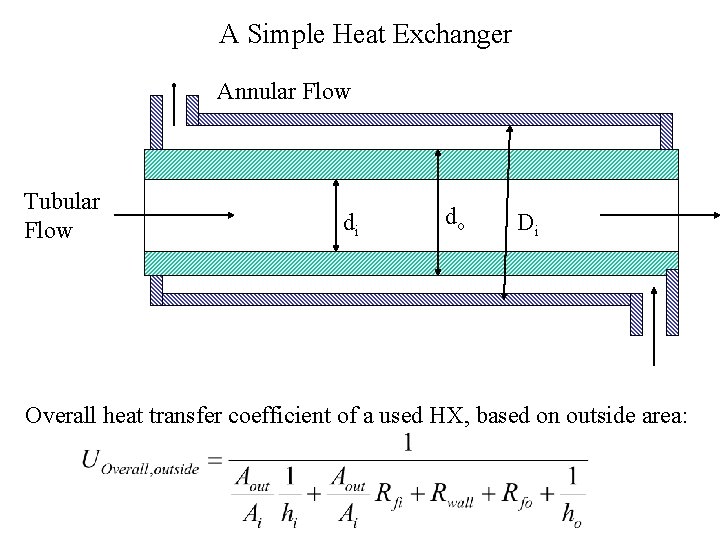
A Simple Heat Exchanger Annular Flow Tubular Flow di do Di Overall heat transfer coefficient of a used HX, based on outside area:

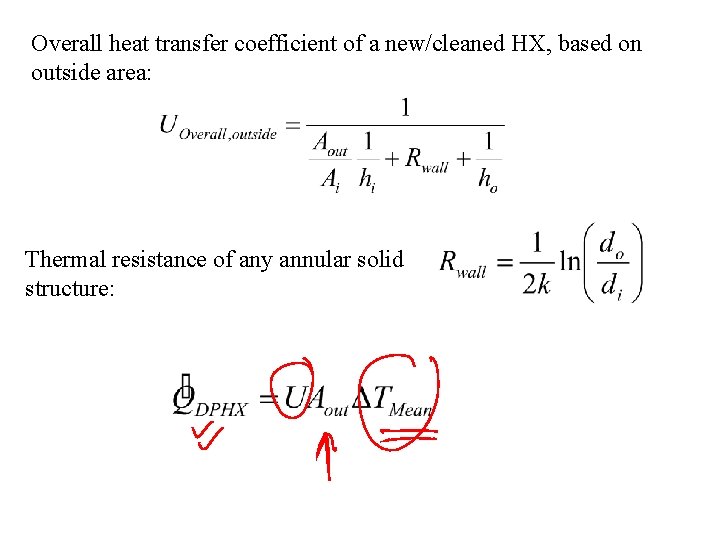
Overall heat transfer coefficient of a new/cleaned HX, based on outside area: Thermal resistance of any annular solid structure:

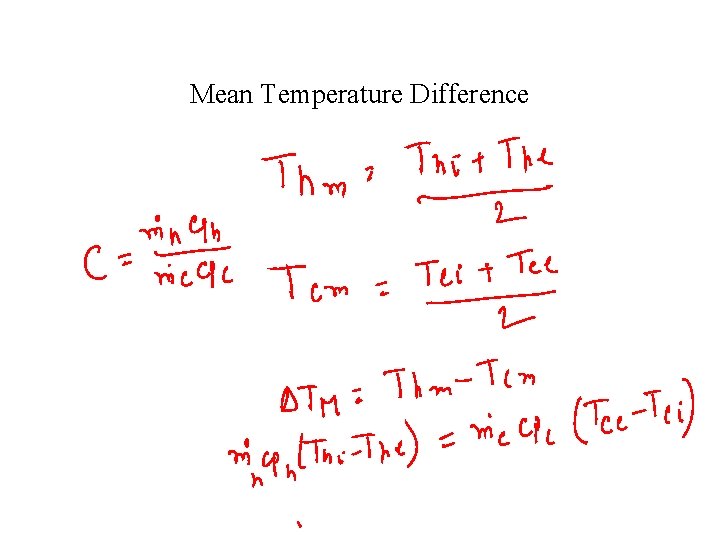
Mean Temperature Difference

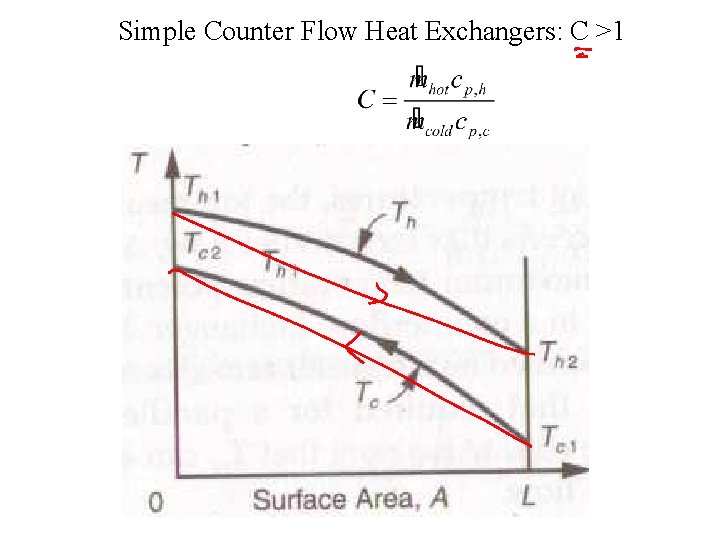
Simple Counter Flow Heat Exchangers: C >1

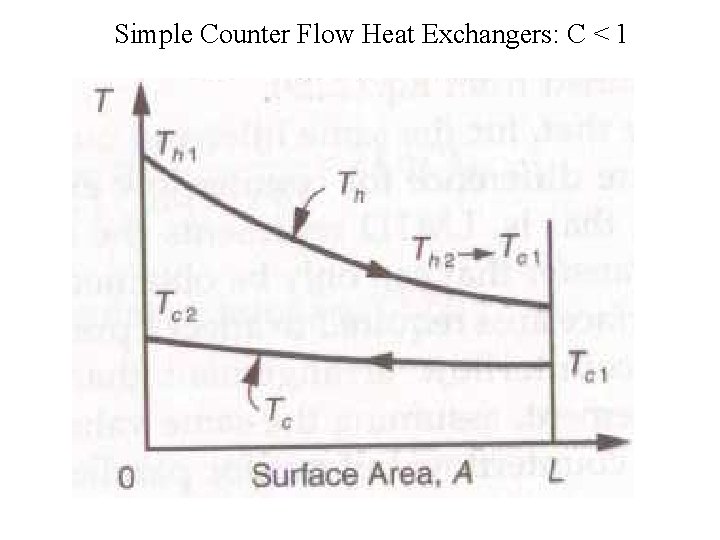
Simple Counter Flow Heat Exchangers: C < 1

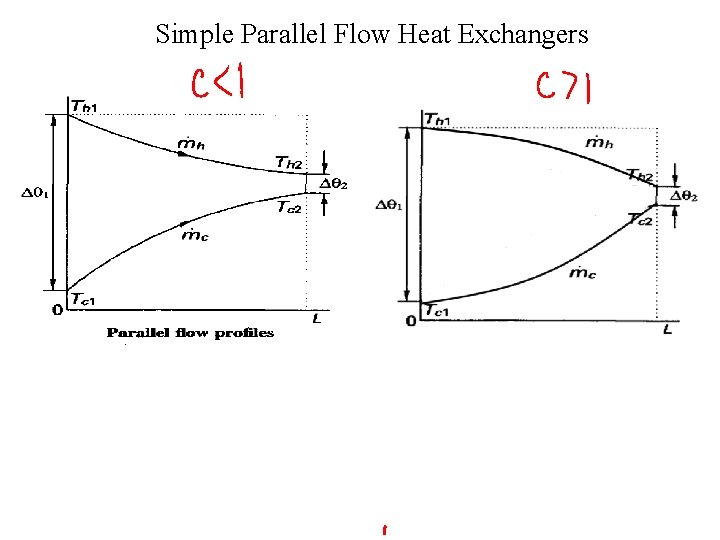
Simple Parallel Flow Heat Exchangers

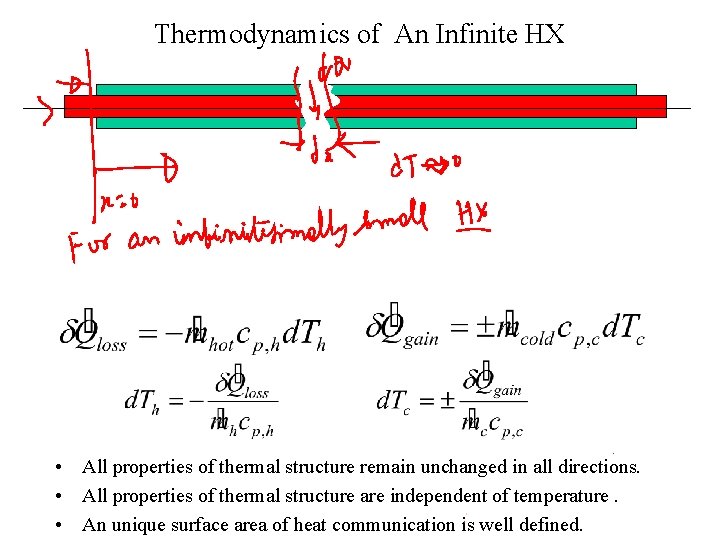
Thermodynamics of An Infinite HX • All properties of thermal structure remain unchanged in all directions. • All properties of thermal structure are independent of temperature. • An unique surface area of heat communication is well defined.

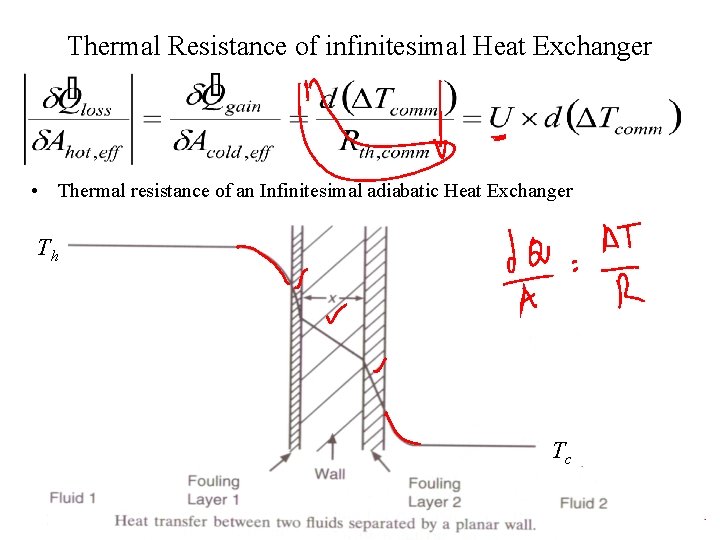
Thermal Resistance of infinitesimal Heat Exchanger • Thermal resistance of an Infinitesimal adiabatic Heat Exchanger Th Tc

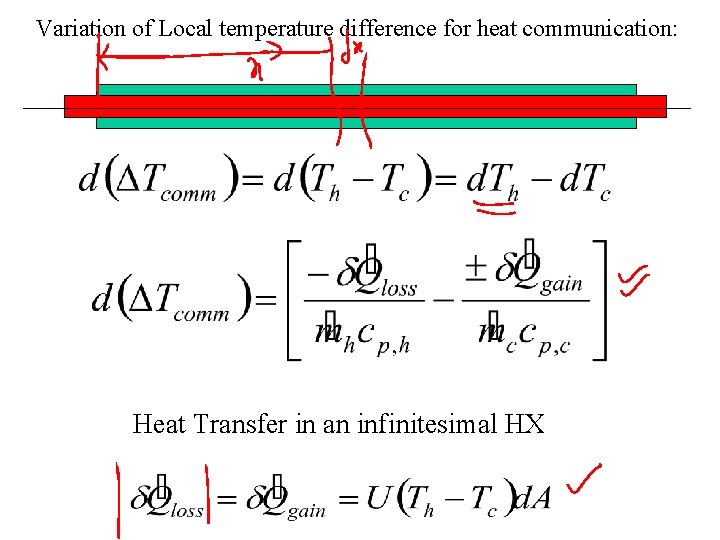
Variation of Local temperature difference for heat communication: Heat Transfer in an infinitesimal HX

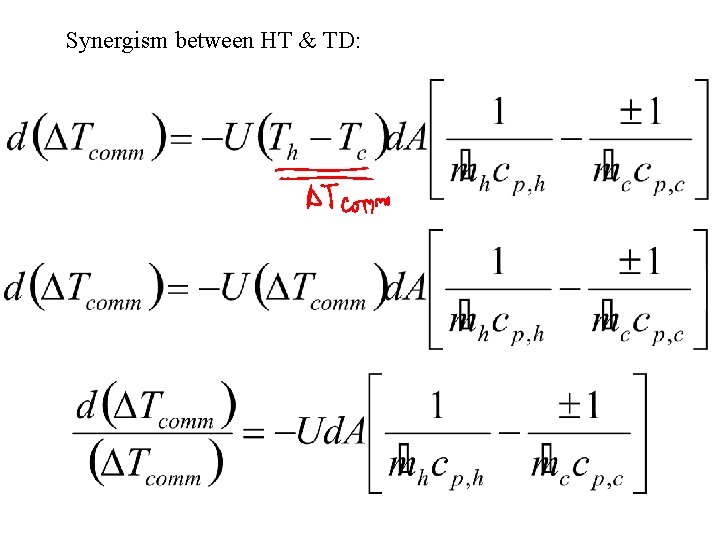
Synergism between HT & TD:

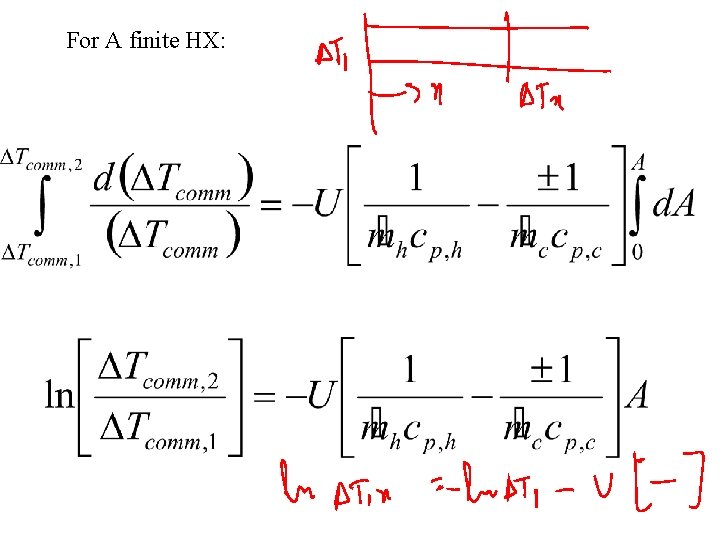
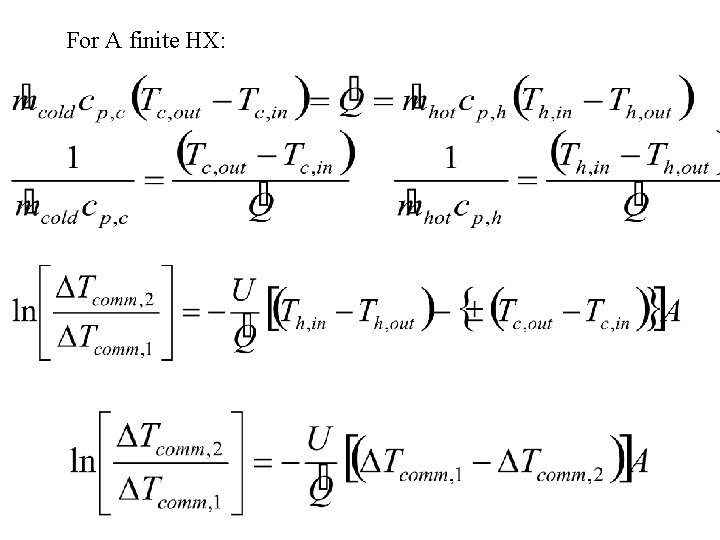
For A finite HX:

For A finite HX:

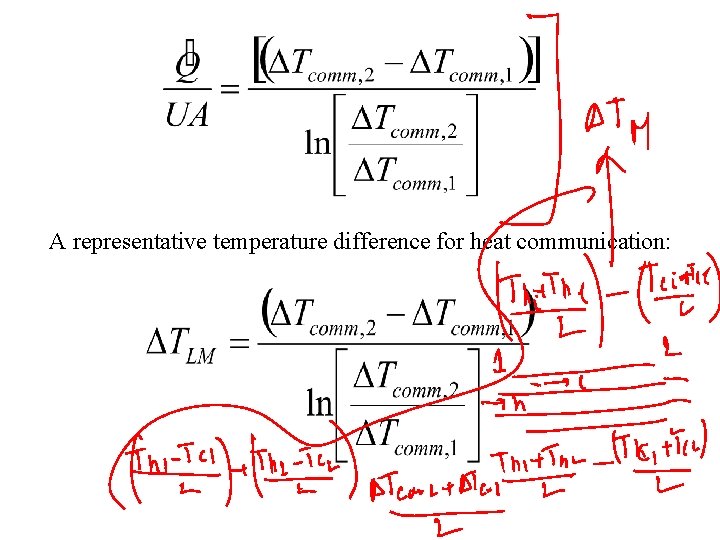
A representative temperature difference for heat communication:

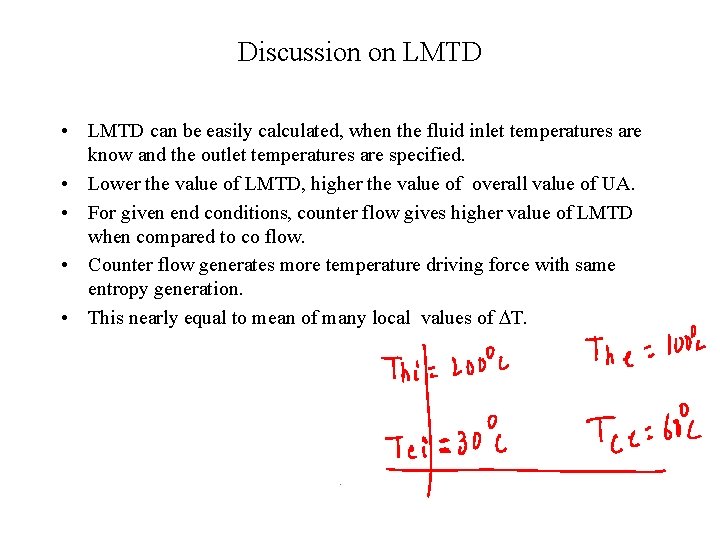
Discussion on LMTD • LMTD can be easily calculated, when the fluid inlet temperatures are know and the outlet temperatures are specified. • Lower the value of LMTD, higher the value of overall value of UA. • For given end conditions, counter flow gives higher value of LMTD when compared to co flow. • Counter flow generates more temperature driving force with same entropy generation. • This nearly equal to mean of many local values of DT.
 Shell bundle clearance
Shell bundle clearance Michael rijssenbeek
Michael rijssenbeek Heat rejection equipment
Heat rejection equipment Ddi heat exchangers inc
Ddi heat exchangers inc Scraped surface heat exchangers in food industry
Scraped surface heat exchangers in food industry Are heat exchangers isobaric
Are heat exchangers isobaric Ddi heat exchangers
Ddi heat exchangers Floating head heat exchanger parts
Floating head heat exchanger parts Fundamental chemical laws
Fundamental chemical laws Differential form of gauss’s law in magneto statics is
Differential form of gauss’s law in magneto statics is Fundamental laws of magnetostatics
Fundamental laws of magnetostatics Charles de secondat
Charles de secondat Fundamental of heat and mass transfer
Fundamental of heat and mass transfer Debugging by deduction
Debugging by deduction Judge past tense
Judge past tense Modals of deduction and possibility
Modals of deduction and possibility Natural deduction cheat sheet
Natural deduction cheat sheet Argument styles
Argument styles Forms of inductive reasoning
Forms of inductive reasoning Rule based deduction system in artificial intelligence
Rule based deduction system in artificial intelligence Inference and deduction
Inference and deduction Obligation and deduction
Obligation and deduction Permissible deduction
Permissible deduction Natural deduction cheat sheet
Natural deduction cheat sheet What is deductive logic
What is deductive logic Régularisation coefficient déduction tva comptabilisation
Régularisation coefficient déduction tva comptabilisation Induction v deduction
Induction v deduction Deduction practice pictures
Deduction practice pictures Cost recovery deduction
Cost recovery deduction Sequential covering algorithm in data mining
Sequential covering algorithm in data mining Offset multiplier
Offset multiplier Induction as inverted deduction
Induction as inverted deduction Gst deduction at source
Gst deduction at source What is utr in gst
What is utr in gst What is inductive argument
What is inductive argument Sound vs unsound arguments
Sound vs unsound arguments Deductive method
Deductive method