Cyclic Structure of Fructose As a ketohexose fructose



- Slides: 21

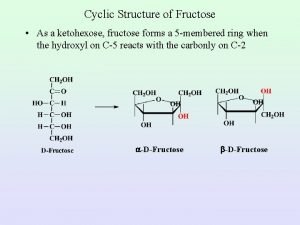
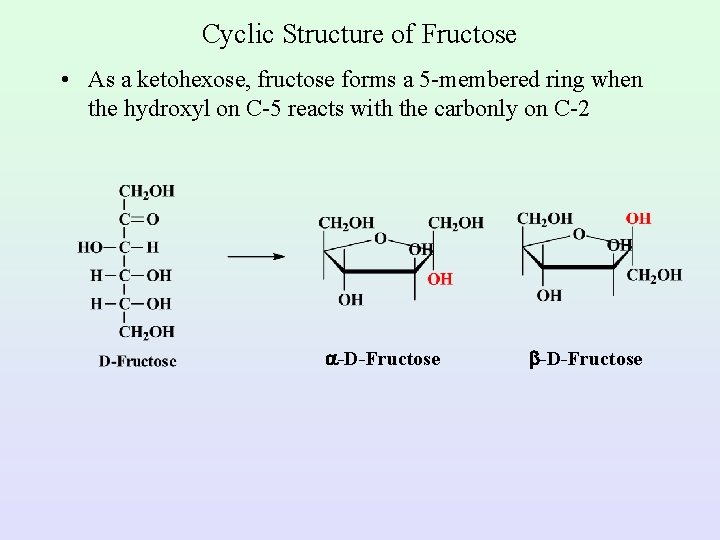
Cyclic Structure of Fructose • As a ketohexose, fructose forms a 5 -membered ring when the hydroxyl on C-5 reacts with the carbonly on C-2 -D-Fructose

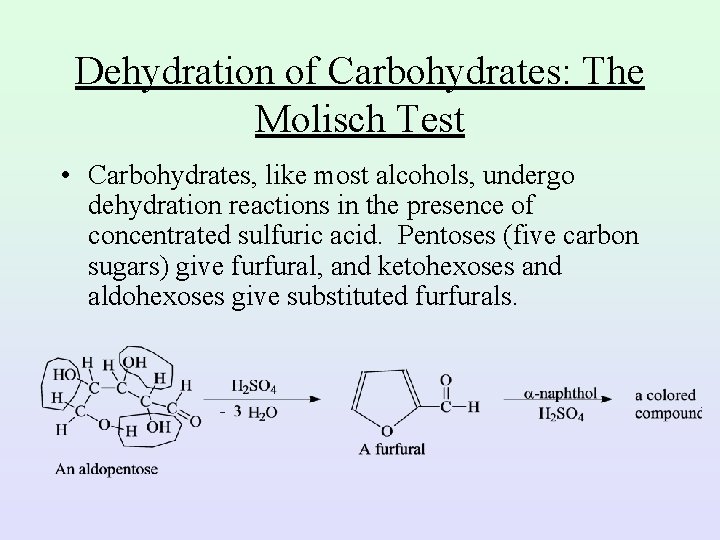
Dehydration of Carbohydrates: The Molisch Test • Carbohydrates, like most alcohols, undergo dehydration reactions in the presence of concentrated sulfuric acid. Pentoses (five carbon sugars) give furfural, and ketohexoses and aldohexoses give substituted furfurals.

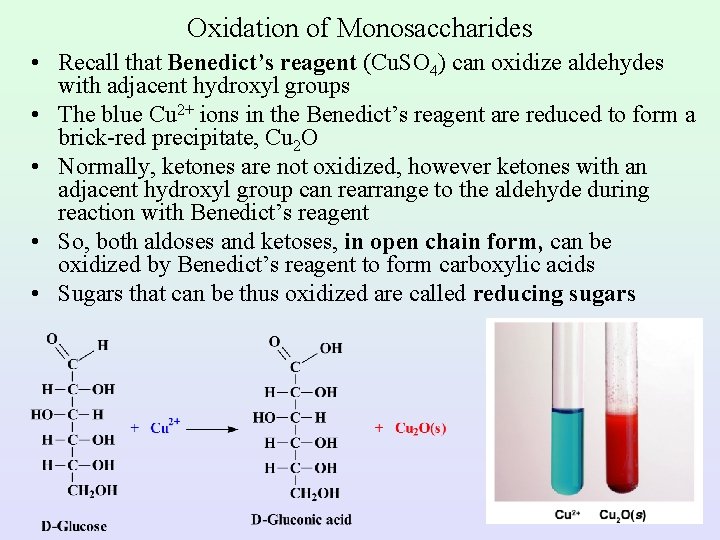
Oxidation of Monosaccharides • Recall that Benedict’s reagent (Cu. SO 4) can oxidize aldehydes with adjacent hydroxyl groups • The blue Cu 2+ ions in the Benedict’s reagent are reduced to form a brick-red precipitate, Cu 2 O • Normally, ketones are not oxidized, however ketones with an adjacent hydroxyl group can rearrange to the aldehyde during reaction with Benedict’s reagent • So, both aldoses and ketoses, in open chain form, can be oxidized by Benedict’s reagent to form carboxylic acids • Sugars that can be thus oxidized are called reducing sugars

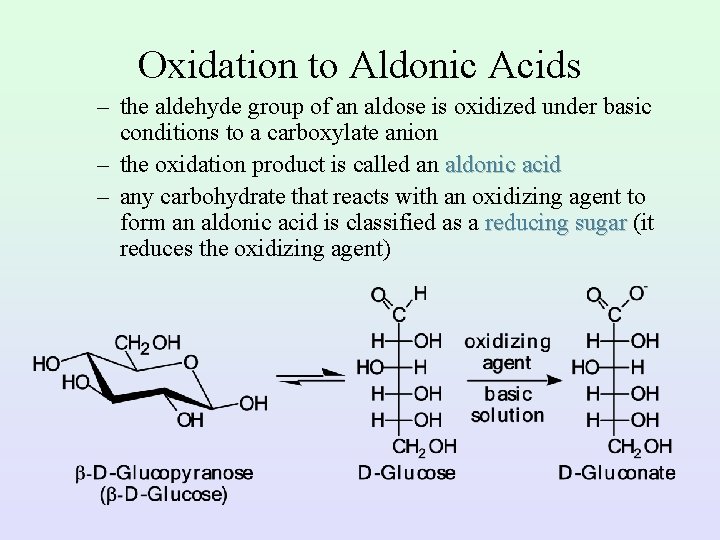
Oxidation to Aldonic Acids – the aldehyde group of an aldose is oxidized under basic conditions to a carboxylate anion – the oxidation product is called an aldonic acid – any carbohydrate that reacts with an oxidizing agent to form an aldonic acid is classified as a reducing sugar (it reduces the oxidizing agent)

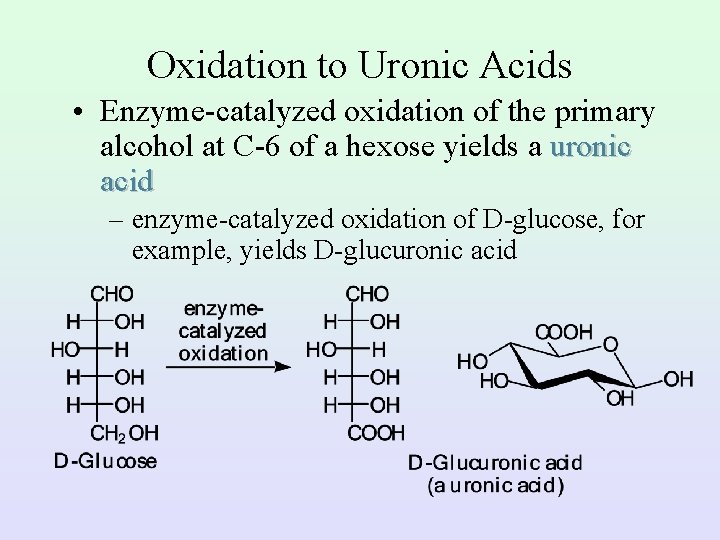
Oxidation to Uronic Acids • Enzyme-catalyzed oxidation of the primary alcohol at C-6 of a hexose yields a uronic acid – enzyme-catalyzed oxidation of D-glucose, for example, yields D-glucuronic acid

D-Glucuronic Acid – D-glucuronic acid is widely distributed in the plant and animal world – in humans, it is an important component of the acidic polysaccharides of connective tissues – it is used by the body to detoxify foreign phenols and alcohols; in the liver, these compounds are converted to glycosides of glucuronic acid and excreted in the urine

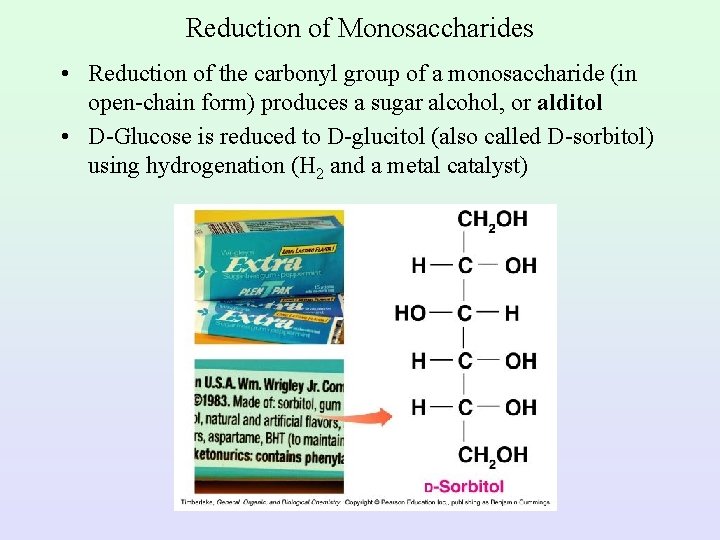
Reduction of Monosaccharides • Reduction of the carbonyl group of a monosaccharide (in open-chain form) produces a sugar alcohol, or alditol • D-Glucose is reduced to D-glucitol (also called D-sorbitol) using hydrogenation (H 2 and a metal catalyst)

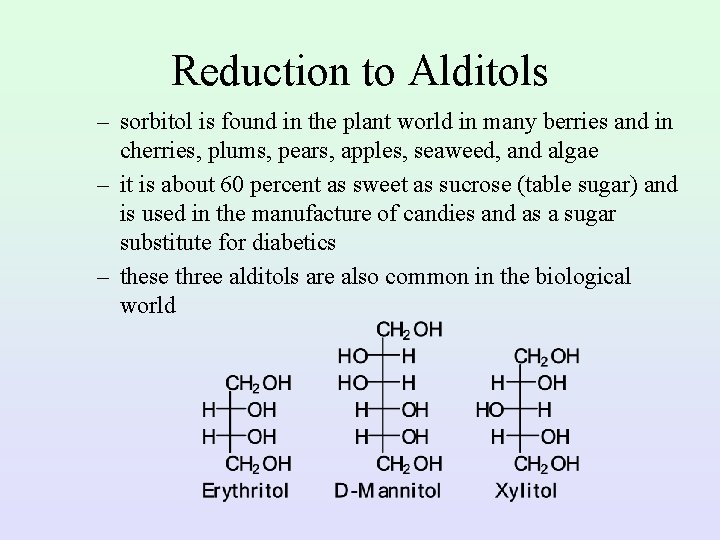
Reduction to Alditols – sorbitol is found in the plant world in many berries and in cherries, plums, pears, apples, seaweed, and algae – it is about 60 percent as sweet as sucrose (table sugar) and is used in the manufacture of candies and as a sugar substitute for diabetics – these three alditols are also common in the biological world

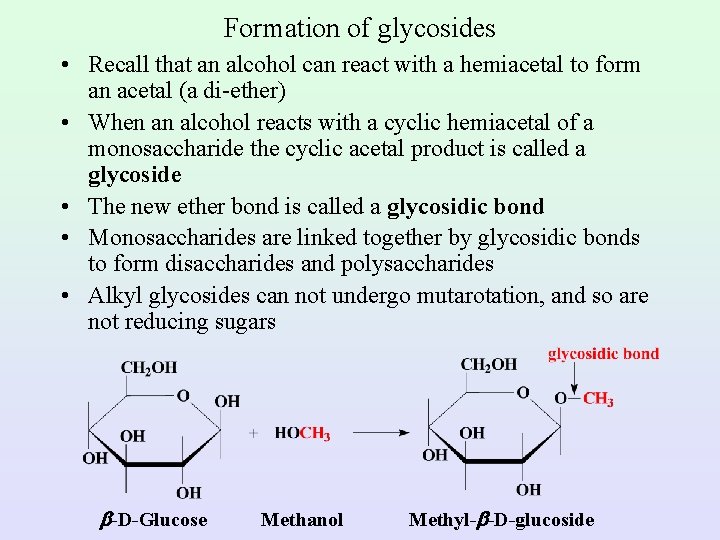
Formation of glycosides • Recall that an alcohol can react with a hemiacetal to form an acetal (a di-ether) • When an alcohol reacts with a cyclic hemiacetal of a monosaccharide the cyclic acetal product is called a glycoside • The new ether bond is called a glycosidic bond • Monosaccharides are linked together by glycosidic bonds to form disaccharides and polysaccharides • Alkyl glycosides can not undergo mutarotation, and so are not reducing sugars -D-Glucose Methanol Methyl- -D-glucoside

Formation of Glycosides – a cyclic acetal derived from a monosaccharide is called a glycoside – the bond from the anomeric carbon to the -OR group is called a glycosidic bond – mutarotation is not possible in a glycoside because an acetal, unlike a hemiacetal, is not in equilibrium with the open-chain carbonyl-containing compound – glycosides are stable in water and aqueous base, but like other acetals, are hydrolyzed in aqueous acid to an alcohol and a monosaccharide – glycosides are named by listing the alkyl or aryl group bonded to oxygen followed by the name of the carbohydrate in which the ending -e is replaced by -ide

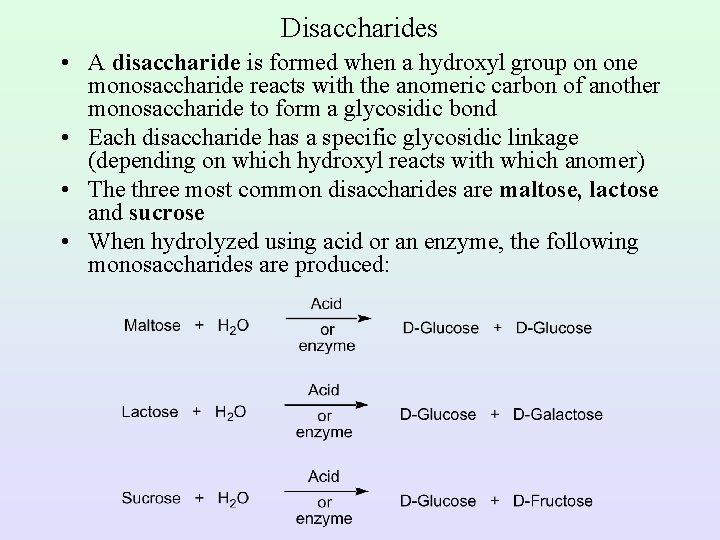
Disaccharides • A disaccharide is formed when a hydroxyl group on one monosaccharide reacts with the anomeric carbon of another monosaccharide to form a glycosidic bond • Each disaccharide has a specific glycosidic linkage (depending on which hydroxyl reacts with which anomer) • The three most common disaccharides are maltose, lactose and sucrose • When hydrolyzed using acid or an enzyme, the following monosaccharides are produced:

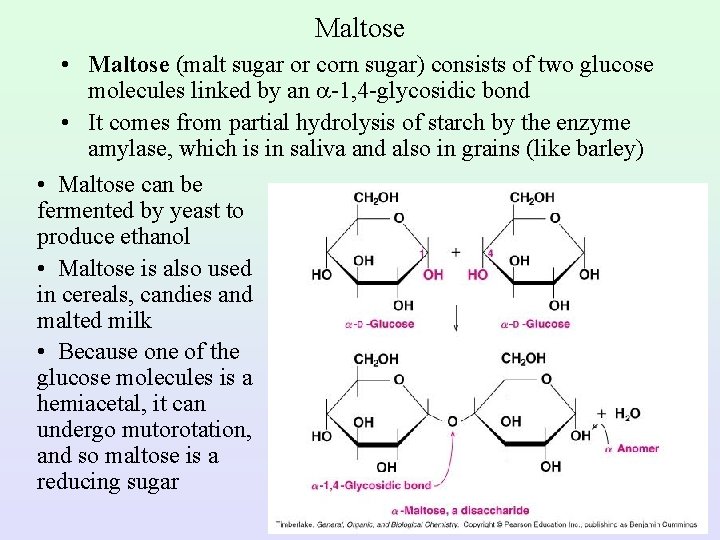
Maltose • Maltose (malt sugar or corn sugar) consists of two glucose molecules linked by an -1, 4 -glycosidic bond • It comes from partial hydrolysis of starch by the enzyme amylase, which is in saliva and also in grains (like barley) • Maltose can be fermented by yeast to produce ethanol • Maltose is also used in cereals, candies and malted milk • Because one of the glucose molecules is a hemiacetal, it can undergo mutorotation, and so maltose is a reducing sugar

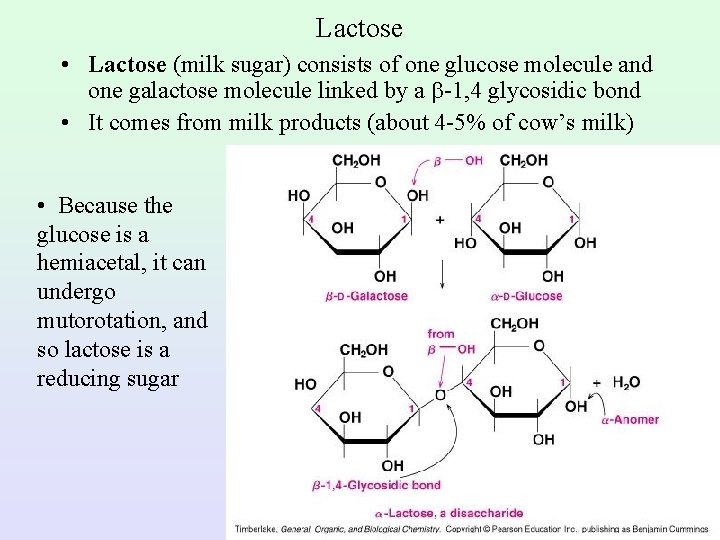
Lactose • Lactose (milk sugar) consists of one glucose molecule and one galactose molecule linked by a -1, 4 glycosidic bond • It comes from milk products (about 4 -5% of cow’s milk) • Because the glucose is a hemiacetal, it can undergo mutorotation, and so lactose is a reducing sugar

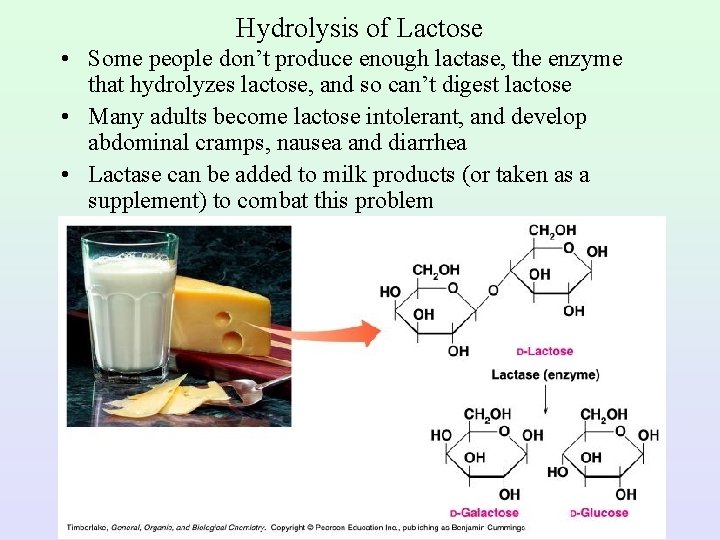
Hydrolysis of Lactose • Some people don’t produce enough lactase, the enzyme that hydrolyzes lactose, and so can’t digest lactose • Many adults become lactose intolerant, and develop abdominal cramps, nausea and diarrhea • Lactase can be added to milk products (or taken as a supplement) to combat this problem

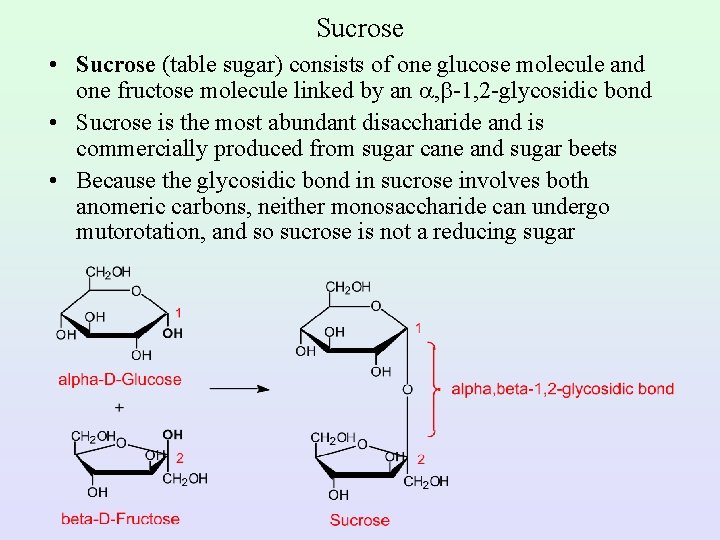
Sucrose • Sucrose (table sugar) consists of one glucose molecule and one fructose molecule linked by an , -1, 2 -glycosidic bond • Sucrose is the most abundant disaccharide and is commercially produced from sugar cane and sugar beets • Because the glycosidic bond in sucrose involves both anomeric carbons, neither monosaccharide can undergo mutorotation, and so sucrose is not a reducing sugar

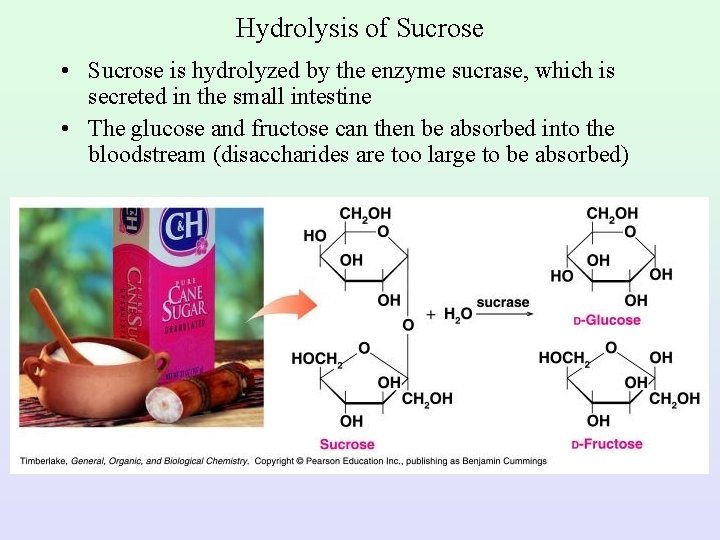
Hydrolysis of Sucrose • Sucrose is hydrolyzed by the enzyme sucrase, which is secreted in the small intestine • The glucose and fructose can then be absorbed into the bloodstream (disaccharides are too large to be absorbed)

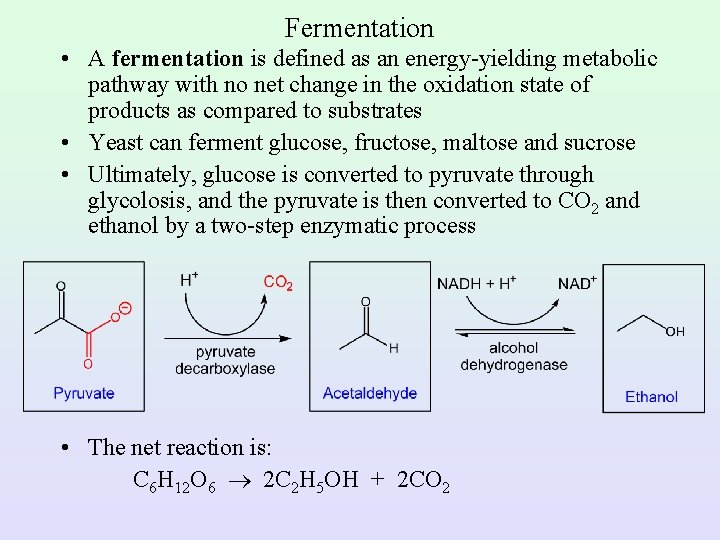
Fermentation • A fermentation is defined as an energy-yielding metabolic pathway with no net change in the oxidation state of products as compared to substrates • Yeast can ferment glucose, fructose, maltose and sucrose • Ultimately, glucose is converted to pyruvate through glycolosis, and the pyruvate is then converted to CO 2 and ethanol by a two-step enzymatic process • The net reaction is: C 6 H 12 O 6 2 C 2 H 5 OH + 2 CO 2

Polysaccharides • A polysaccharide is a polymer consisting of hundreds to thousands of monosaccharides joined together by glycosidic linkages • Three biologically important polysaccharides are starch, glycogen and cellulose - all three are polymers of D-glucose, but they differ in the type of glycosidic bond and/or the amount of branching • Starch and glycogen are used for storage of carbohydrates - starch is found in plants and glycogen in animals - the polymers take up less room than would the individual glucose molecules, so are more efficient for storage • Cellulose is a structural material used in formation of cell walls in plants

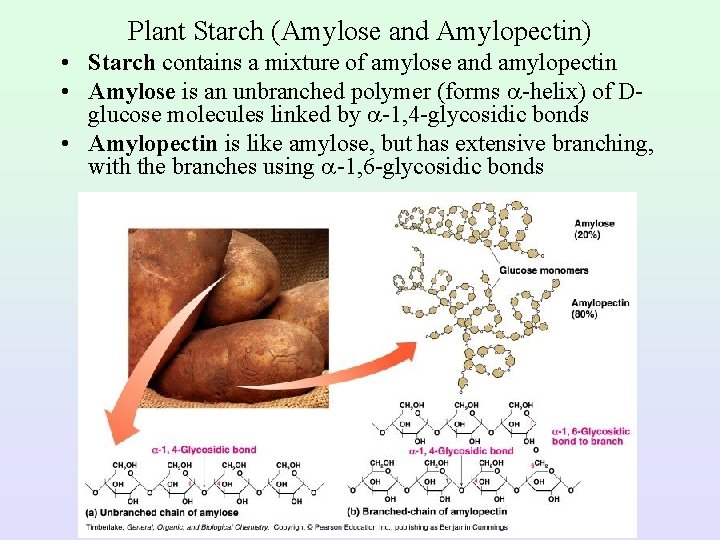
Plant Starch (Amylose and Amylopectin) • Starch contains a mixture of amylose and amylopectin • Amylose is an unbranched polymer (forms -helix) of Dglucose molecules linked by -1, 4 -glycosidic bonds • Amylopectin is like amylose, but has extensive branching, with the branches using -1, 6 -glycosidic bonds

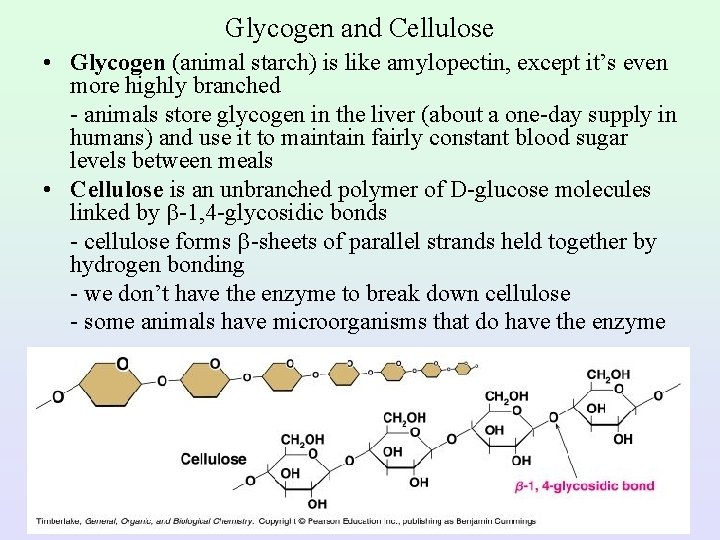
Glycogen and Cellulose • Glycogen (animal starch) is like amylopectin, except it’s even more highly branched - animals store glycogen in the liver (about a one-day supply in humans) and use it to maintain fairly constant blood sugar levels between meals • Cellulose is an unbranched polymer of D-glucose molecules linked by -1, 4 -glycosidic bonds - cellulose forms -sheets of parallel strands held together by hydrogen bonding - we don’t have the enzyme to break down cellulose - some animals have microorganisms that do have the enzyme

Iodine Test for Starch • The presence of starch can easily be identified using iodine (I 2) • Rows of iodine atoms form in the core of the -helix of amylose, forming a dark blue complex • Because amylopectin, glycogen and cellulose do not form helices, they do not complex well with iodine, so do not show the blue color (they show a purple or brown color) • Monosaccharides do not interact with the iodine, so no color is produced
 Fructose cyclic structure
Fructose cyclic structure D-glyceraldehyde fischer projection
D-glyceraldehyde fischer projection Metabolism of fructose and galactose
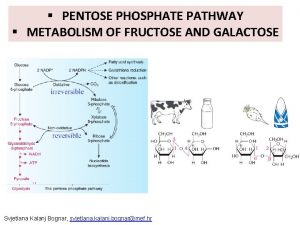
Metabolism of fructose and galactose Cyclisation of fructose
Cyclisation of fructose Qualitative analysis of carbohydrates practical
Qualitative analysis of carbohydrates practical Cyclisation of fructose
Cyclisation of fructose Moles to grams
Moles to grams Hexokinase phosphofructokinase and pyruvate kinase
Hexokinase phosphofructokinase and pyruvate kinase Fructose cyclization
Fructose cyclization Zuckerstammbaum
Zuckerstammbaum Oreo glycemic index
Oreo glycemic index Metobolic encephalopathy
Metobolic encephalopathy Ketose sugar
Ketose sugar Sperm fructose
Sperm fructose Apetimen syrup fructose
Apetimen syrup fructose Is fructose ionic or covalent
Is fructose ionic or covalent Sperm fructose
Sperm fructose Glucose galactose mannose
Glucose galactose mannose Sperm fructose
Sperm fructose Fructose urea
Fructose urea Fructose chiralitätszentren
Fructose chiralitätszentren Fructose
Fructose