Counting Atoms Chemistry is a quantitative science we




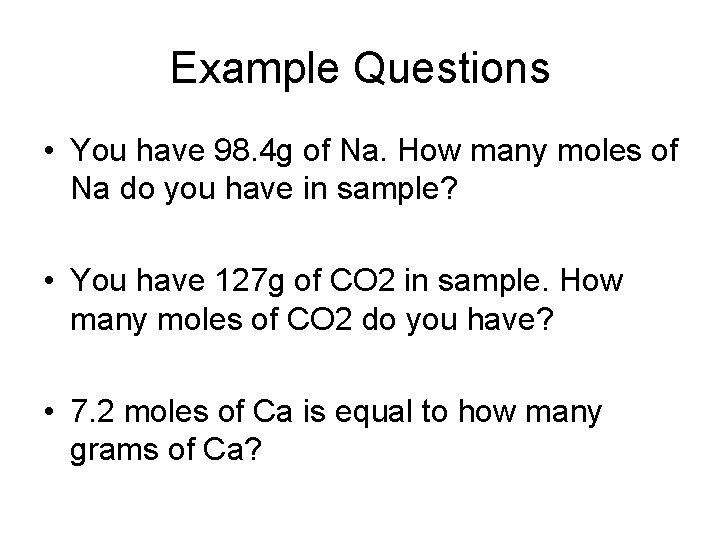








- Slides: 21

Counting Atoms • Chemistry is a quantitative science - we need a "counting unit. " • The MOLE • 1 mole is the amount of substance that contains as many particles (atoms or molecules) as there are in 12. 0 g of C-12.

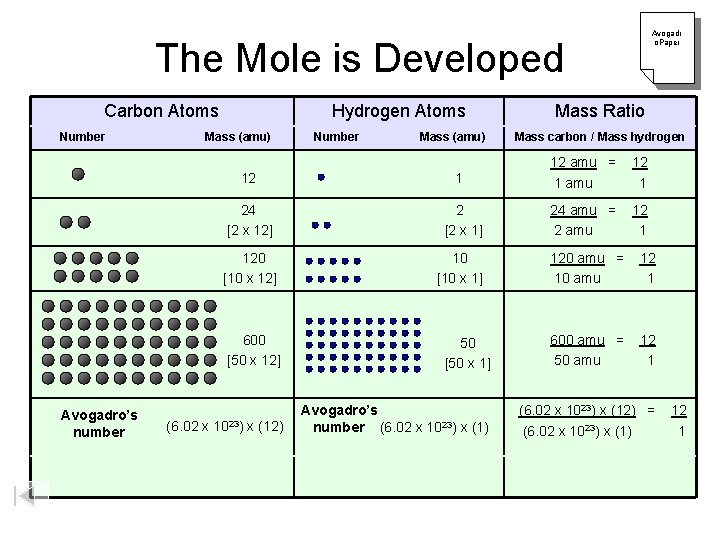
The Mole is Developed Carbon Atoms Number Mass (amu) 12 24 Hydrogen Atoms Number Mass (amu) Avogadr o. Paper Mass Ratio Mass carbon / Mass hydrogen 12 amu = 12 1 1 amu 1 [2 x 12] 24 amu = 12 [2 x 1] 2 amu 1 120 [10 x 12] 10 120 amu = 12 [10 x 1] 10 amu 1 600 [50 x 12] 600 amu = 12 50 [50 x 1] 50 amu 1 Avogadro’s (6. 02 x 1023) x (12) number Avogadro’s number (6. 02 x 1023) x (1) (6. 02 x 1023) x (12) = 12 (6. 02 x 1023) x (1) 1

Particles in a Mole Amadeo Avogadro Amedeo Avogadro (1766 -1856) never knew his own number; it was named in his honor by a French scientist in 1909. its value was first estimated by Josef Loschmidt, an Austrian (1776 – 1856) chemistry teacher, in 1895. ? quadrillions trillions billions thousands millions 1 mole = 60221367360000000 or 6. 022 x 1023 There is Avogadro's number of particles in a mole of any substance.

Careers in Chemistry - Philosopher Q: How much is a mole? A: A mole is a quantity used by chemists to count atoms and molecules. A mole of something is equal to 6. 02 x 1023 “somethings. ” 1 mole = 602 200 000 000 000 Q: Can you give me an example to put that number in perspective? A: A computer that can count 10, 000 atoms per second would take 2, 000, 000 years to count 1 mole of a substance.

Counting to 1 Mole Is that right? A computer counting 10 million atoms every second would need to count for 2 billion years to count just a single mole. Lets look at the mathematics. x sec = 1 year 365 days 24 hours 60 min 60 sec = 31, 536, 000 sec 1 year 1 day 1 hour 1 min Therefore 1 year has 31, 536, 000 seconds or 3. 1536 x 107 sec. A computer counting 10, 000 atoms every second could count 3. 153 x 1014 atoms every year. Finally, 6. 02 x 1023 atoms divided by 3. 1536 x 1014 atoms every year equals 1, 908, 929, 477 years or approximately 2 billion years!

How Big is a Mole? One mole of marbles would cover the entire Earth (oceans included) for a depth of three miles. One mole of $100 bills stacked one on top of another would reach from the Sun to Pluto and back 7. 5 million times. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills.

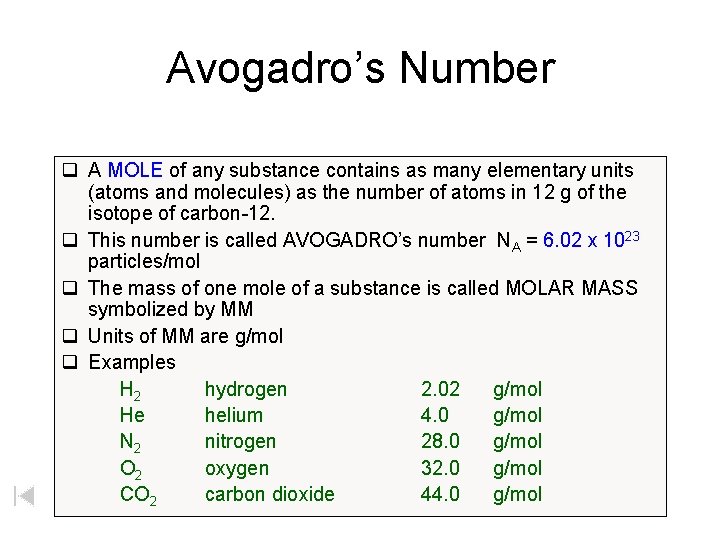
Avogadro’s Number q A MOLE of any substance contains as many elementary units (atoms and molecules) as the number of atoms in 12 g of the isotope of carbon-12. q This number is called AVOGADRO’s number NA = 6. 02 x 1023 particles/mol q The mass of one mole of a substance is called MOLAR MASS symbolized by MM q Units of MM are g/mol q Examples H 2 hydrogen 2. 02 g/mol He helium 4. 0 g/mol N 2 nitrogen 28. 0 g/mol O 2 oxygen 32. 0 g/mol CO 2 carbon dioxide 44. 0 g/mol

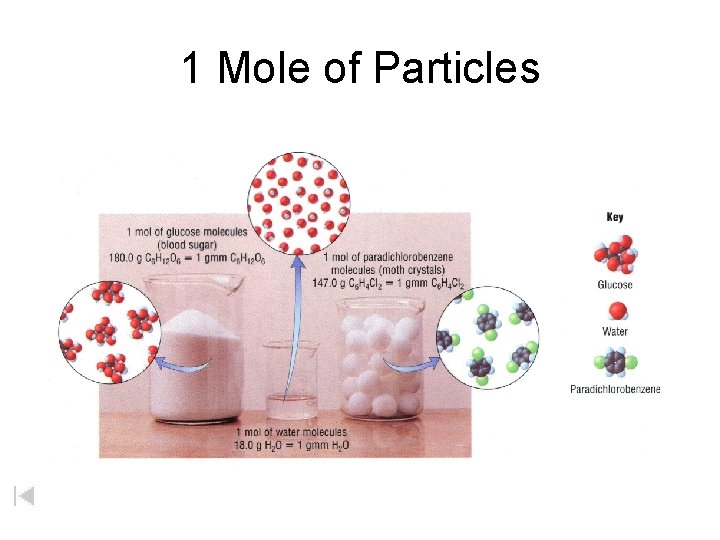
1 Mole of Particles
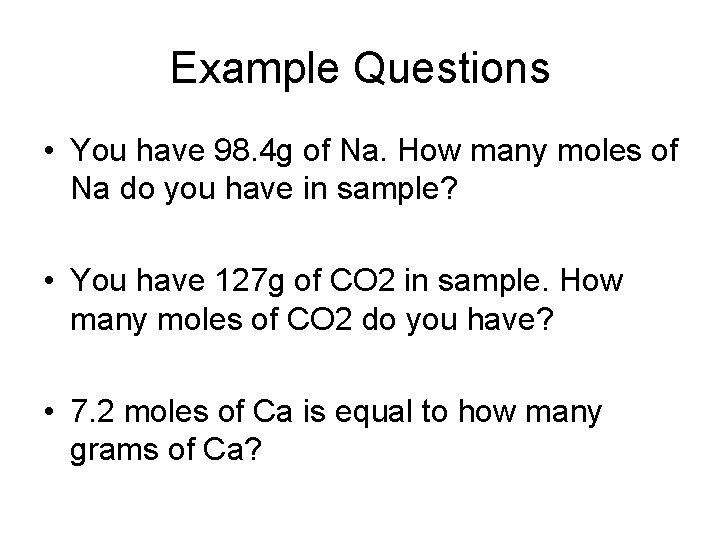
Example Questions • You have 98. 4 g of Na. How many moles of Na do you have in sample? • You have 127 g of CO 2 in sample. How many moles of CO 2 do you have? • 7. 2 moles of Ca is equal to how many grams of Ca?

Example questions •

Example Questions •

Limiting reactant • The reactant in a chemical reaction that limits the amount of product that can be formed. The reaction will stop when all of the limiting reactant is consumed.

Excess Reactant • Excess Reactant - The reactant in a chemical reaction that remains when a reaction stops when the limiting reactant is completely consumed. The excess reactant remains because there is nothing with which it can react.

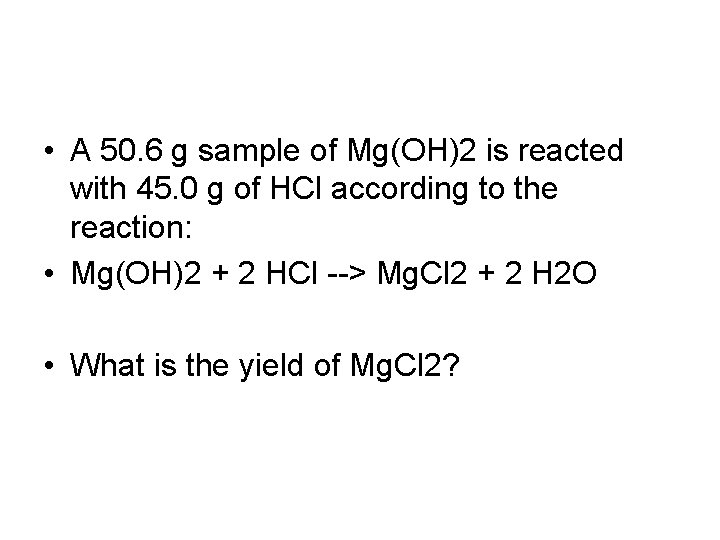
• A 50. 6 g sample of Mg(OH)2 is reacted with 45. 0 g of HCl according to the reaction: • Mg(OH)2 + 2 HCl --> Mg. Cl 2 + 2 H 2 O • What is the yield of Mg. Cl 2?

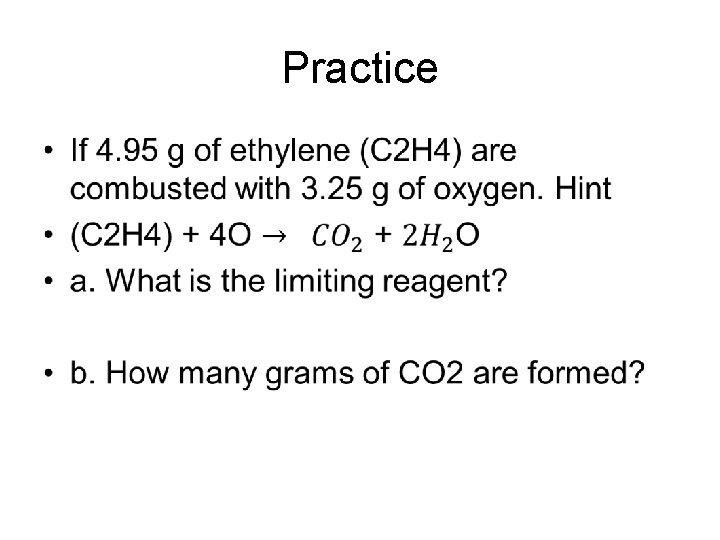
Practice •

Practice •

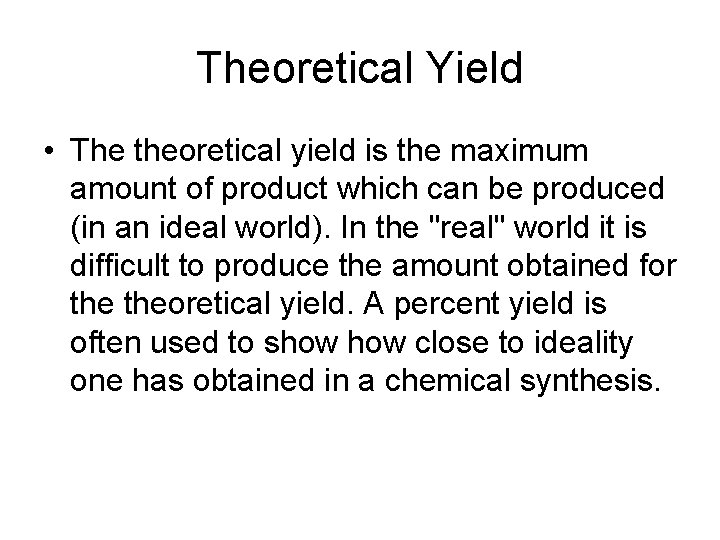
Theoretical Yield • The theoretical yield is the maximum amount of product which can be produced (in an ideal world). In the "real" world it is difficult to produce the amount obtained for theoretical yield. A percent yield is often used to show close to ideality one has obtained in a chemical synthesis.

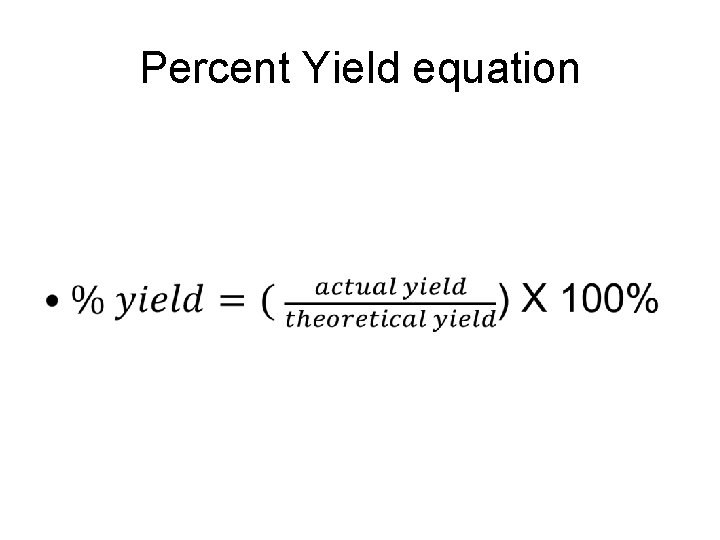
Percent Yield equation •

Practice problems • Experimentally the chemist found that 46. 5 g of Na. Cl was collected at the end of the experiment. However in his calculations he found that he should have produced 50 g of Na. Cl. What was his percentage yield?

Practice problems • Given the reaction: • 2 H + O H 2 O • In this reaction you have 16 grams of both H and O. After the reaction you collected 16. 98 grams H 2 O what is your percentage yield?

Practice • NH 3 + O 2 → NO + H 2 O • Assuming you had 43 grams of NH 3 and 23 grams O 2. You actually collected 71. 23 grams of NO. What was your percentage yield of NO?