Counting Atoms Chemistry is a quantitative science we





- Slides: 9

Counting Atoms • Chemistry is a quantitative science - we need a "counting unit. " • The MOLE • 1 mole is the amount of substance that contains as many particles (atoms or molecules) as there are in 12. 0 g of C-12.

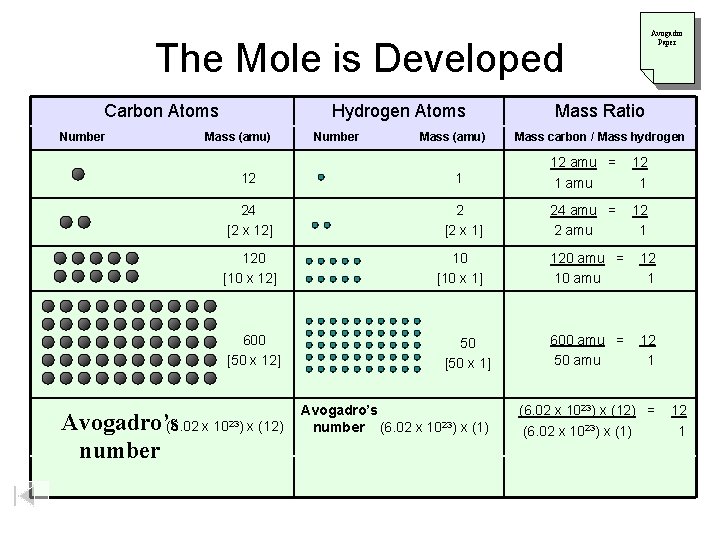
Avogadro Paper The Mole is Developed Carbon Atoms Number Hydrogen Atoms Mass (amu) 12 Mass (amu) 1 Mass carbon / Mass hydrogen 12 amu = 1 amu 12 1 24 [2 x 12] 2 [2 x 1] 24 amu = 2 amu 120 [10 x 12] 10 [10 x 1] 120 amu = 10 amu 12 1 600 amu = 50 amu 12 1 600 [50 x 12] Avogadro’s (6. 02 x number Number Mass Ratio 1023) x (12) 50 [50 x 1] Avogadro’s number (6. 02 x 1023) x (1) (6. 02 x 1023) x (12) = (6. 02 x 1023) x (1) 12 1

How Big is a Mole? One mole of marbles would cover the entire Earth (oceans included) for a depth of two miles. One mole of $1 bills stacked one on top of another would reach from the Sun to Pluto and back 7. 5 million times. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills.

Particles in a Mole Amedeo Avogadro (1776 – 1856) ? quadrillions trillions billions thousands millions 1 mole = 60221367360000000 or 6. 022 x 1023 There is Avogadro's number of particles in a mole of any substance.

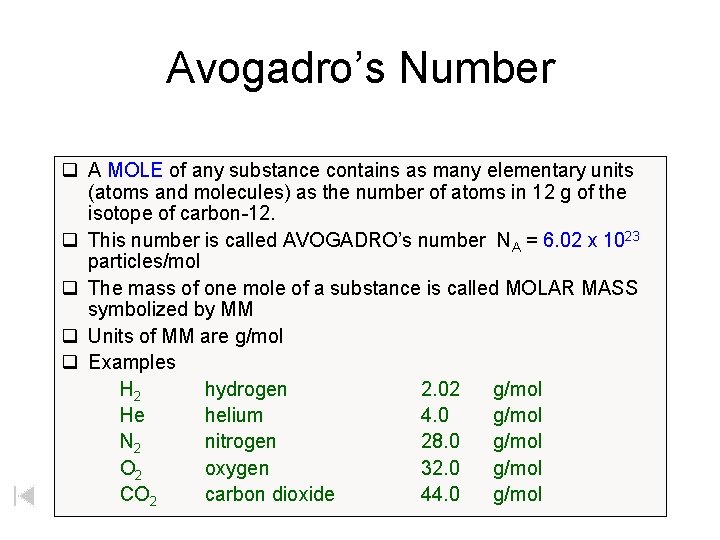
Avogadro’s Number q A MOLE of any substance contains as many elementary units (atoms and molecules) as the number of atoms in 12 g of the isotope of carbon-12. q This number is called AVOGADRO’s number NA = 6. 02 x 1023 particles/mol q The mass of one mole of a substance is called MOLAR MASS symbolized by MM q Units of MM are g/mol q Examples H 2 hydrogen 2. 02 g/mol He helium 4. 0 g/mol N 2 nitrogen 28. 0 g/mol O 2 oxygen 32. 0 g/mol CO 2 carbon dioxide 44. 0 g/mol

Molecular Weight and Molar Mass Molecular weight is the sum of atomic weights of all atoms in the molecule. example: Na. Cl has a molecular weight of 58. 5 a. m. u. this is composed of a single molecule of Na. Cl Molar mass = molecular weight in grams. example: Na. Cl has a molar mass of 58. 5 grams this is composed of a 6. 02 x 1023 molecules of Na. Cl

The Molar Mass and Number of Particles in One-Mole Quantities Substance Molar Mass Number of Particles in One Mole Carbon (C) 12. 0 g 6. 02 x 1023 C atoms Sodium (Na) 23. 0 g 6. 02 x 1023 Na atoms Iron (Fe) 55. 9 g 6. 02 x 1023 Fe atoms Na. F (preventative for dental cavities) 42. 0 g 6. 02 x 1023 Na. F formula units Ca. CO 3 (antacid) 100. 1 g 6. 02 x 1023 Ca. CO 3 formula units C 6 H 12 O 6 (glucose) 180. 0 g 6. 02 x 1023 glucose molecules C 8 H 10 N 4 O 2 (caffeine) 194. 0 g 6. 02 x 1023 caffeine molecules

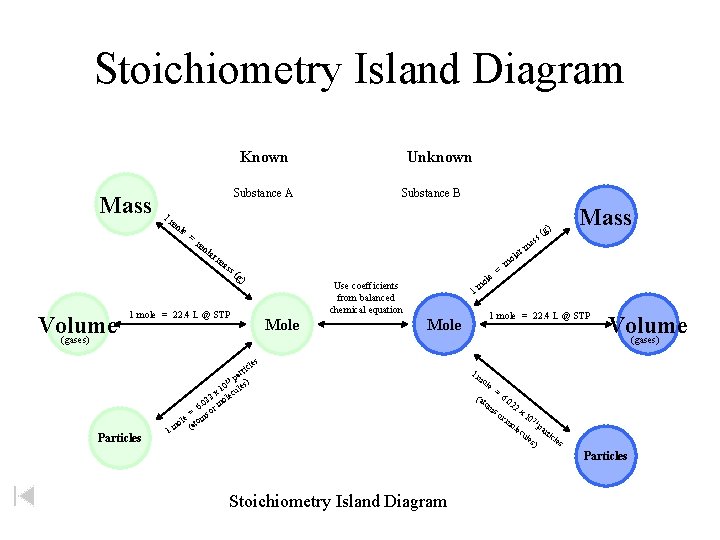
Stoichiometry Island Diagram Known Mass Substance A Substance B 1 m ole = ) mo lar Volume (gases) Unknown ma ss rm ola (g ) ole 1 m Use coefficients from balanced chemical equation 1 mole = 22. 4 L @ STP Mole = g s( Mass as m 1 mole = 22. 4 L @ STP Mole Volume (gases) s Particles cle rti a p ) 23 s 10 cule x e 22 ol 6. 0 or m = s ole (atom m 1 1 m ole (at om = 6. 0 22 x 1 rm 0 23 ole pa r cu les ticle s ) so Particles Stoichiometry Island Diagram

Stoichiometry Island Diagram Known Unknown Substance A Substance B M Mass Mountain Mass Liter Lagoon V Volume Mole Volume Particles P Particle Place Stoichiometry Island Diagram