Colligative properties Solutions Part 4 Electrolytes a quick







- Slides: 16

Colligative properties Solutions Part 4

Electrolytes: a quick review • Electrolytes form ions in solution. • Ions allow water to conduct electric current • Three types of electrolytes: strong , weak, and nonelectrolytes.

Strong Electrolytes • Strong electrolytes ionize completely in water. • More ions in the water, more conductivity. • Strong electrolytes include: – Strong acids HCl H+ + Cl– Strong bases Na. OH Na+ + OH– Soluble salts Ca. Cl 2 Ca 2+ + 2 Cl-

Molarity: review • Moles per liter • 1. 0 M = 1 mole solute per 1 liter solution. • Question • What is the molarity of 1. 56 g of gaseous HCl in a 26. 8 m. L solution?

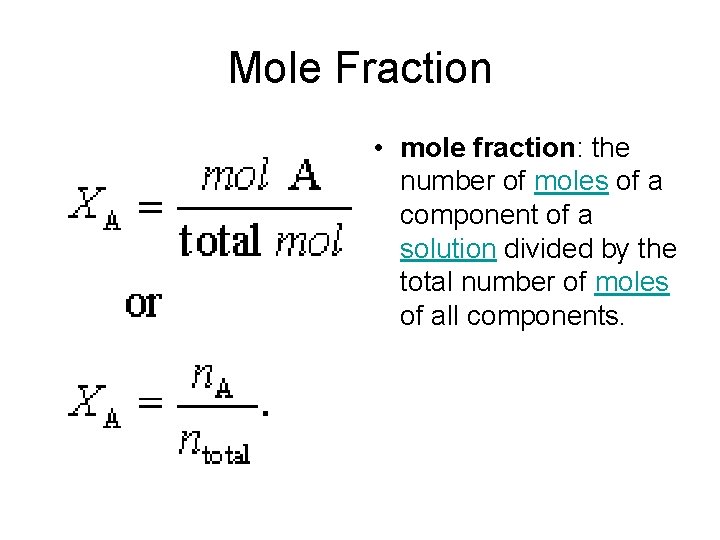
Mole Fraction • mole fraction: the number of moles of a component of a solution divided by the total number of moles of all components.

Example: • 58. 5 grams of Na. Cl are in a one liter solution. • What is the Molarity of the Na. Cl? • 1 M • 58. 5 grams of Na. Cl are in 1800 grams of water. • What is the mole fraction of Na. Cl? • 1/101

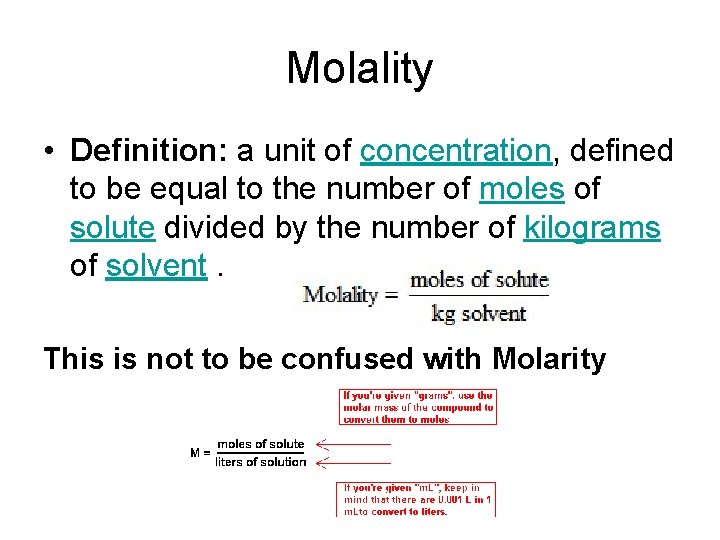
Molality • Definition: a unit of concentration, defined to be equal to the number of moles of solute divided by the number of kilograms of solvent. This is not to be confused with Molarity

Why so you add salt to an icy sidewalk? • • It melts the ice. How? Colligative properties. Why so some types of salt seem to work better than melting the ice than others. • Why? • Colligative properties.

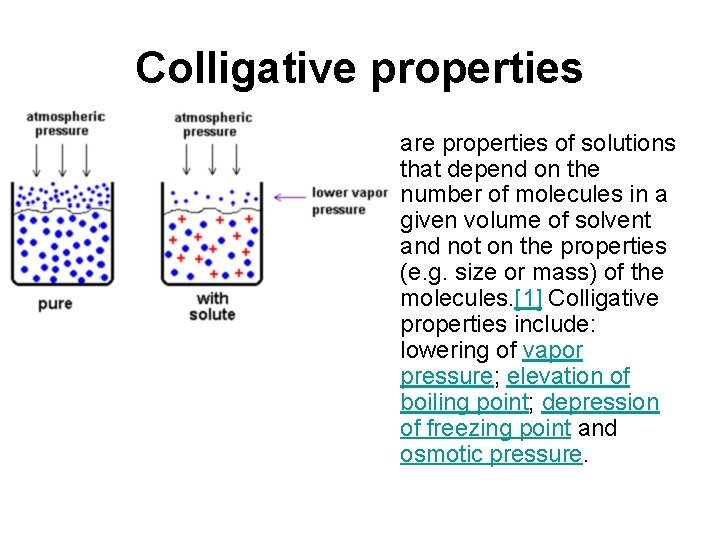
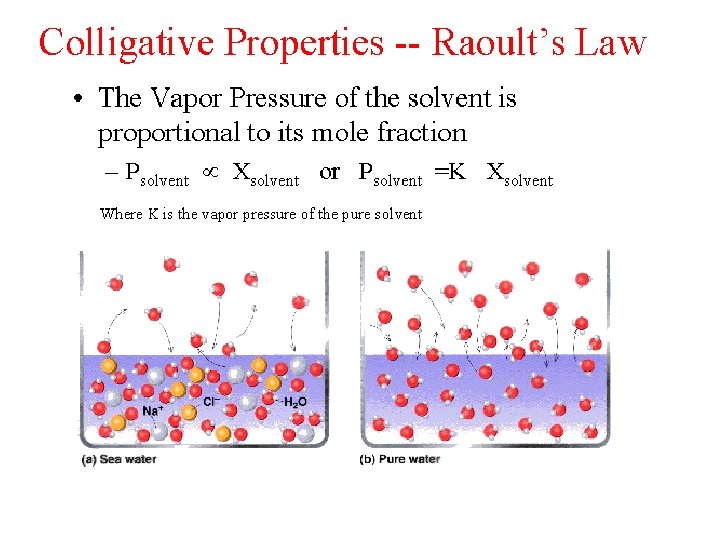
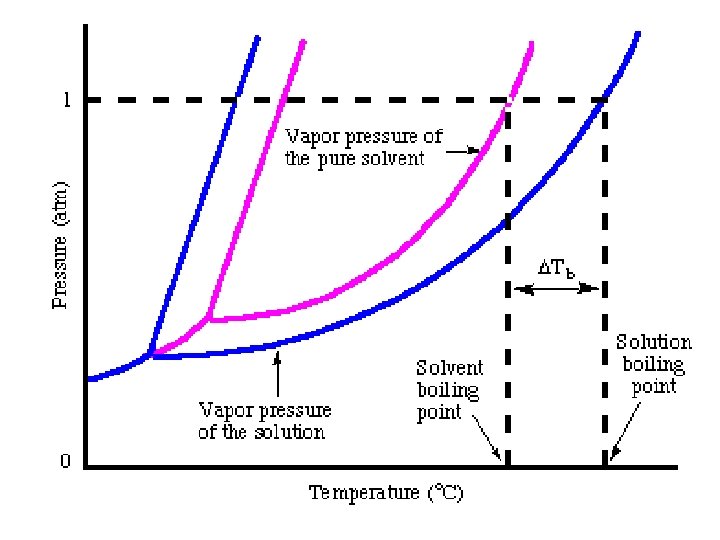
Colligative properties • are properties of solutions that depend on the number of molecules in a given volume of solvent and not on the properties (e. g. size or mass) of the molecules. [1] Colligative properties include: lowering of vapor pressure; elevation of boiling point; depression of freezing point and osmotic pressure.

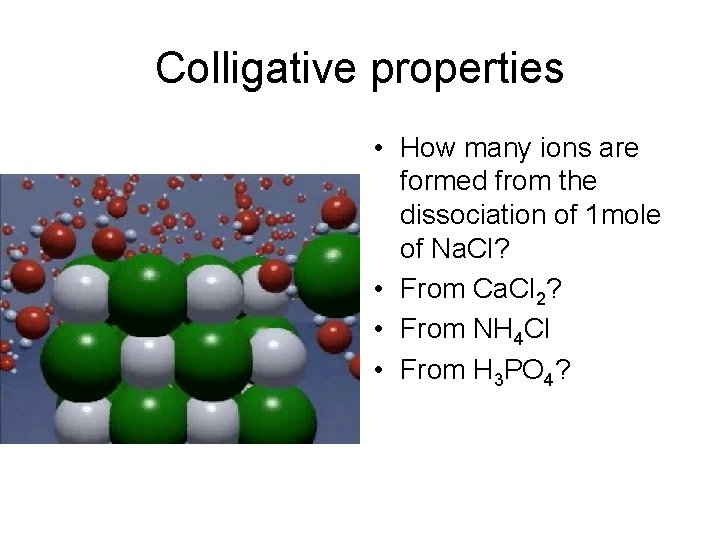
Colligative properties • How many ions are formed from the dissociation of 1 mole of Na. Cl? • From Ca. Cl 2? • From NH 4 Cl • From H 3 PO 4?

Question • What is the concentration of each type of ion in the following solutions? • 0. 50 M Co(NO 3)2 • 1 M Fe(Cl. O 4)3

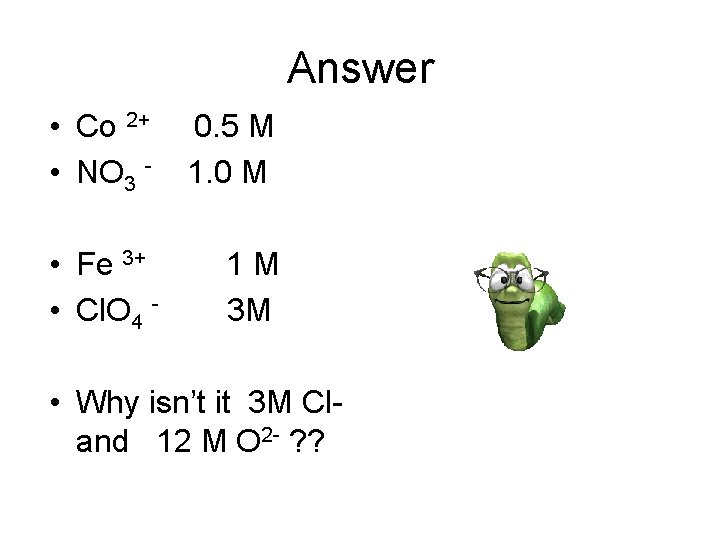
Answer • Co 2+ • NO 3 - 0. 5 M 1. 0 M • Fe 3+ • Cl. O 4 - 1 M 3 M • Why isn’t it 3 M Cland 12 M O 2 - ? ?


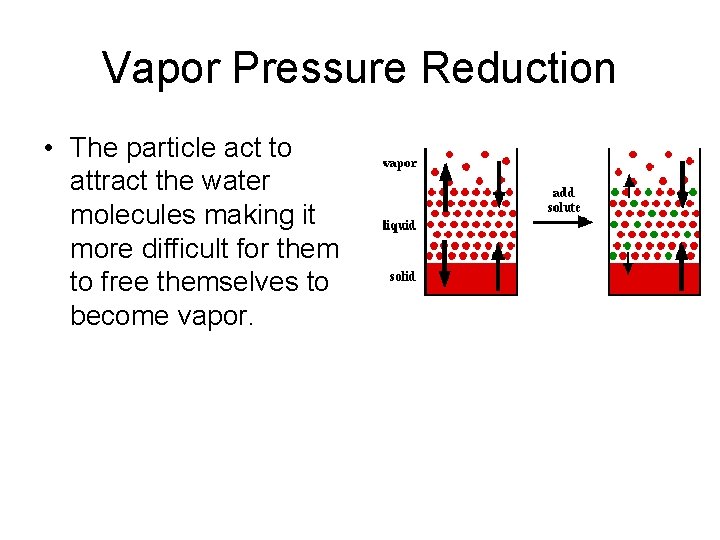
Vapor Pressure Reduction • The particle act to attract the water molecules making it more difficult for them to free themselves to become vapor.

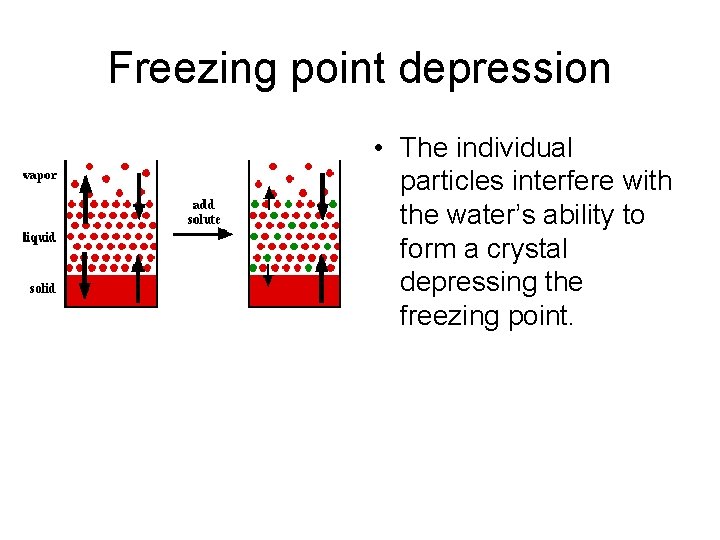
Freezing point depression • The individual particles interfere with the water’s ability to form a crystal depressing the freezing point.
