Cleaning Validation and Critical Cleaning of Topical Drugs






















- Slides: 32

Cleaning Validation and Critical Cleaning of Topical Drugs Dijana Hadziselimovic Technical Services Laboratory Specialist STERIS Corp. Email: Dijana_Hadziselimovic@steris. com April 11, 2018

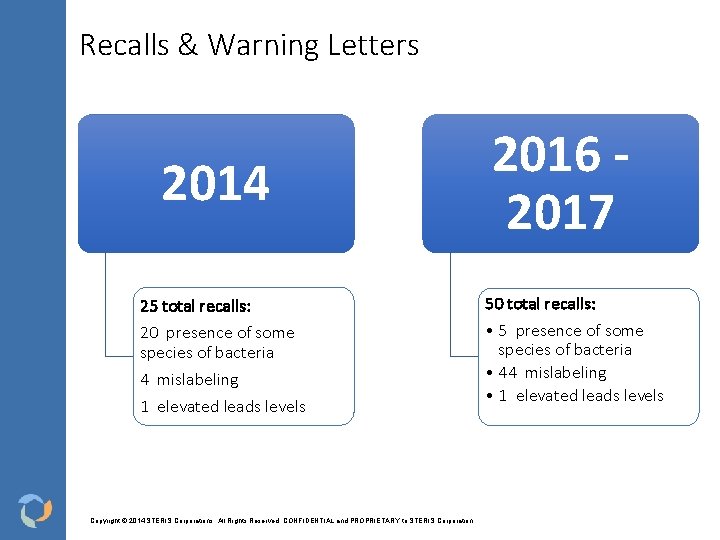
Recalls & Warning Letters 2014 25 total recalls: 20 presence of some species of bacteria 4 mislabeling 1 elevated leads levels Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation 2016 2017 50 total recalls: • 5 presence of some species of bacteria • 44 mislabeling • 1 elevated leads levels

Cleaning “The process of removing contaminants from process equipment and maintaining the condition of equipment such that the equipment can be safely used for subsequent product manufacture” A contaminant is the presence of a minor ingredient in another chemical or mixture, often at the trace level Cleanliness is a subjective term and must be defined (acceptance criteria) Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Why clean? • Product integrity • • Cross-contamination Microbial integrity Other chemical species Lot integrity • Equipment reuse • Regulatory compliance Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Traditional Approach to Validation • • • Documented evidence at commercial scale (protocols) High level of assurance (data/reports) Consistency (minimum of 3 lots) Pre-established quality attributes (acceptance criteria) Summary and package closure For repeated cleaning SOP’s DESIGN >>> VALIDATION >>> MONITORING Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

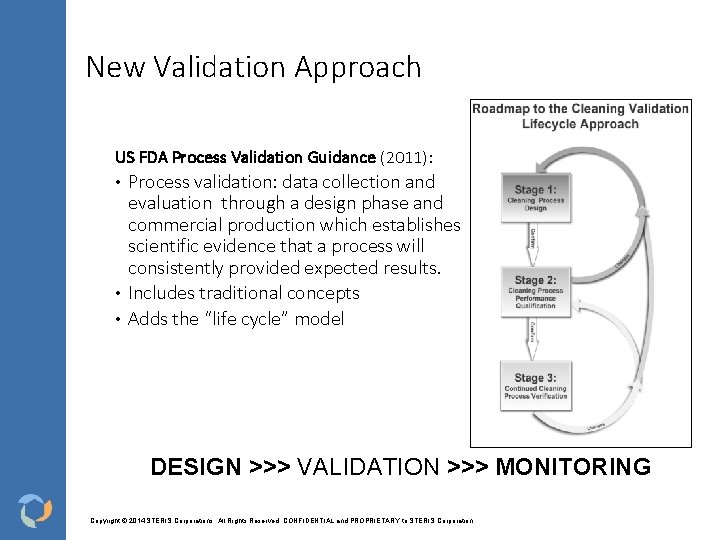
New Validation Approach US FDA Process Validation Guidance (2011): Process validation: data collection and evaluation through a design phase and commercial production which establishes scientific evidence that a process will consistently provided expected results. • Includes traditional concepts • Adds the “life cycle” model • DESIGN >>> VALIDATION >>> MONITORING Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Cleaning process design • Key elements to be considered in design • Equipment to be cleaned • Soils to be removed • Cleaning methods • Cleaning agents • Cleaning mechanisms • Cleaning parameters • Residue limits Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Equipment • Type and design? • Difficult to clean locations • Is selection of cleaning method limited? • Legacy systems for CIP? • Materials of construction? • Important for selection of cleaning agents and parameters • Clean individually or as train Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Soils • Actives, excipients, process materials, bioburden, endotoxin • Amounts of soils on surfaces • Nature of soils on surfaces • • • Freshly deposited Dried on during process Dried on during dirty hold time Baked on during process Compacted Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Methods • Extent of automation • Extent of disassembly • Examples • • • Fixed CIP Portable CIP Parts washer Ultrasonic Manual (soak, brush, wipe, spray) Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Cleaning Agents Screening • Chemistry • Performance • Rinsability • Substrate compatibility • Supplier qualification • Environmental health & safety • Stability • Toxicity • Technical Support • Global availability Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

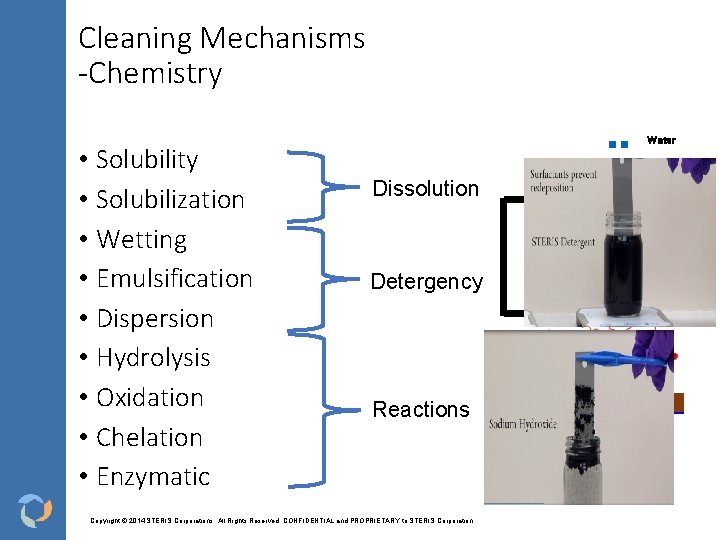
Cleaning Mechanisms -Chemistry • Solubility • Solubilization • Wetting • Emulsification • Dispersion • Hydrolysis • Oxidation • Chelation • Enzymatic Water Surfactant Residue Dissolution Detergency Reactions Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation Surface

Residue limits • What residues will I be testing for? • How clean is clean? • How low? • Analytical methods used for measuring residues can perhaps affect selection of cleaning process, but is not typically a limiting factor Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

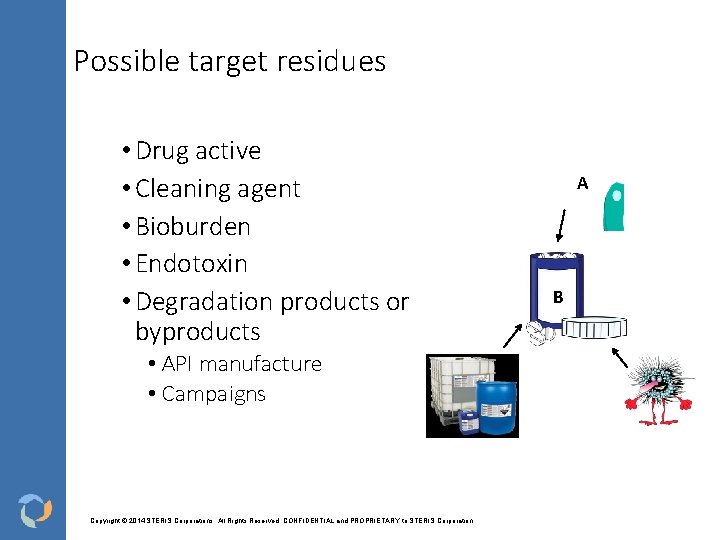
Possible target residues • Drug active • Cleaning agent • Bioburden • Endotoxin • Degradation products or byproducts • API manufacture • Campaigns Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation A B

What’s an acceptable level? • Multiple approaches • Based on potential effects of target residue on subsequent product • Possible effects & issues • • Pharmacology of residue Toxicity of residue Dosing of next drug product Stability issues • May utilize a “safety” factor Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Acceptance criteria Finished drug products • Traditional approach based on: • Fourmen and Mullen (for actives) • Most stringent of dose calculation and 10 ppm (in next product), plus • Visually clean • PIC/S approach - most stringent of. . . – – – Dose calculation in next product 10 ppm in next product Visually clean Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Critical cleaning parameters • Time • Action/impingement • Cleaning chemistry • Concentration • Temperature • Mixing/flow/turbulence • Water quality • Rinsing Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Laboratory Studies SS coupons are spiked with sample soil (s). Soil conditions are emulated to the actual manufacturing process. Coupons are exposed to multiple cleaning parameters depending upon project’s objectives. Visually clean? Water break free? Weight change? Analytical tests? Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

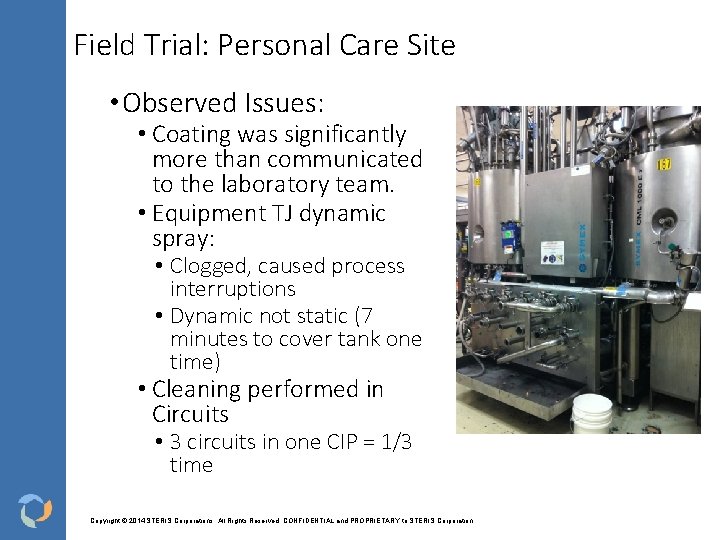
Field Trial: Personal Care Site • Cosmetic company • They were purchasing formulated cleaning agents • Laboratory testing was performed Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Field Trial: Personal Care Site • Determined that 5 % v/v formulated alkaline cleaner with surfactants at 80 °C for 15 minutes effectively cleaned residues. • FAT was performed using three (3) soils: Satin Lips Lip Mask , Bulk Concealer/Lavender CN, TWR Moist SPF 30 Day Cream. Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Field Trial: Personal Care Site • Observed Issues: • Coating was significantly more than communicated to the laboratory team. • Equipment TJ dynamic spray: • Clogged, caused process interruptions • Dynamic not static (7 minutes to cover tank one time) • Cleaning performed in Circuits • 3 circuits in one CIP = 1/3 time Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Field Trial: Personal Care Site • Additional Recommendations • May need to keep pre-rinse in order to keep spray nozzle from clogging and operable during CIP process. • Increase cleaning time • Clean each circuit for the recommended amount of time instead of treating system as a whole. • Still investigating alternative chemistries…. Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Cleaning issue • Foundation with SPF claims containing Titanium Dioxide. • Equipment • Pumps cleaned out of place in parts washer • Current process not able to effectively clean. Equipment soiled with water-proof make-up prior to cleaning Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation Cleaning results after 3% CIP 130 plus 3% Pro. Klenz Booster at 70°C with 5 minute Prewash/15 minute Wash.

Common Issues with Personal Care Sites • Customers will have some of the most difficult soils to clean • Have an understanding of their soil conditions and process. • Restrictions such as: • equipment design/condition • p. H and water temperature limitations (for cleaning and waste water) • may want organic or “green” cleaners (Environmentally friendly, phosphate free, and water restrictions) Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Common Issues with Personal Care Sites • Cost of cleaning agents • Regulated and non regulated products • Cleaning solution coverage • Cleaning pipes (Fluid velocity, dead legs, and drainability). Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Scale-up • Next step is confirmation (or modification) in pilot scale/plant evaluation • • Confirm lab performance Confirm key control parameters Confirm adequate engineering (address flow paths, dead legs, etc. ) Optimize time(s), conditions Determine rinse conditions Identify potential sampling locations Evaluate with analytical method Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Full scale runs • Based upon lab and scale-up studies, as well as data on related cleaning processes, may either: • 1. Perform confirmatory study or studies at full scale (prior to validation runs) • 2. Go immediately to validation protocol run(s) Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Documentation – La b Stu di – Sc ale-u es appl p studi es, if – Co icable m (not mercial scale valid a runs t – An i y sup on) port – Ke data y de c isi prof essio on based nal j udgm on ent Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Cleaning run success • A validation run is deemed acceptable when the equipment is both visibly clean and meets the acceptance criteria for product residues and cleaning agents at the first sampling without additional cleaning required. Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Interim reports • Interim reports must be approved by the cleaning subject matter expert and the Quality Unit. • The validation interim report should include at a minimum the following: − Summary of the activities − Analytical test results − List of all discrepancies and resolutions − Conclusions and recommendations − Approval page Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

ion t a d i l g va n i n a l cle a n i f cts: e l l o The c re e g a k ning pac a e l c the f o y mar m u s and • A s l o c l co oto proto ecuted pr x uns r • All e ns g n i o ean revisi from all cl g records in • Data s of clean s e ie • Cop procedur ing of r r and/o ne monito alidation v ti • Rou ness after rding the a li clean usions reg process. n cl • Con g validatio in clean val page ro • App Final validation package Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation

Dijana Hadziselimovic Technical Services Laboratory Specialist STERIS Corporation Life Sciences Formulated Chemistries E-Mail: dhadzise@steris. com Copyright © 2014 STERIS Corporations. All Rights Reserved. CONFIDENTIAL and PROPRIETARY to STERIS Corporation
 Critical semi critical and non critical instruments
Critical semi critical and non critical instruments Semi-critical
Semi-critical Cleaning validation presentation
Cleaning validation presentation Apic cleaning validation guidelines 2014
Apic cleaning validation guidelines 2014 Compare non-critical readers with critical readers.
Compare non-critical readers with critical readers. What is absorption base
What is absorption base Medication administration 3 pretest
Medication administration 3 pretest Injectable medication administration ati posttest
Injectable medication administration ati posttest Guidelines for writing a literature review
Guidelines for writing a literature review Douche dosage form
Douche dosage form Topical agent meaning
Topical agent meaning Syllabus types
Syllabus types Topical scope in research
Topical scope in research Topical dosage forms
Topical dosage forms Content based syllabus example
Content based syllabus example Translate image
Translate image Topical approach definition
Topical approach definition Pengertian topikal
Pengertian topikal How to prepare to preach the word of god
How to prepare to preach the word of god Evaluation of paste
Evaluation of paste What is doses form
What is doses form Parenteral route
Parenteral route Topical pattern of arrangement
Topical pattern of arrangement Topical approach definition
Topical approach definition Topical scope
Topical scope Vitiligo covermark topical
Vitiligo covermark topical Define topical agents
Define topical agents Ray-dee-og-rah-fee
Ray-dee-og-rah-fee Advantages of functional syllabus
Advantages of functional syllabus Closcript topical cream
Closcript topical cream Types of relevance
Types of relevance Mythological allusions in fahrenheit 451
Mythological allusions in fahrenheit 451 A topical approach to lifespan development
A topical approach to lifespan development