Presentation Outline 2 Lifecycle Approach to Cleaning Validation










































- Slides: 57


Presentation Outline 2 Ø Ø Ø Ø Lifecycle Approach to Cleaning Validation: Overview Regulations, Regulatory Guidelines & Guidance Reason, Definition and Concepts of Cleaning Validation Cleaning Procedure Cleaning agent Microbiology/Sterilization Practices and Procedures n n n n Cleaning Validation Development Protocol Development Sampling Analytical methods Acceptable limits Protocol Execution Report Preparation (Summary and Conclusion)

About Speaker: 3 Mr. Pramote Cholayudth 1969 – 1974: B. Sc. Pharm. (Mahidol University) 1974 – 1980: Bayer Laboratories Limited / Assistant Production Manager 1981 – 1997: OLIC (Thailand) Limited / Production Manager 1998 – 2001: Huachiew Chalermprakiet University / Lecturer 2000 – Current: Biolab / Validation Consultant 2010 – Current: GPO Flu Vaccine Plant / KMUTT Consultant 2012 – Current: PM Consult / General Manager

About Speaker: 4 Ms. Piyaphorn Phichaikam Master of Business Administration (M. B. A. ) degree from Burapha University Bachelors degree from Faculty of Pharmaceutical-Sciences, Prince of Songkla University Experience: Industrial Pharmacist BIOLAB CO. , LTD –GMP Manager KMUTT Consultant –GPO Project (WHO GMP) Thai Meiji Pharmaceutical –Production Manager Now – QA Manager (a local company) and PM Consult GMP & Validation Specialist

5 Lifecycle Approach to Cleaning Validation: Overview Bureau of Veterinary Biologics By: Pramote Cholayudth cpramote 2000@yahoo. com; LINE ID: pc 0976 PM Consult

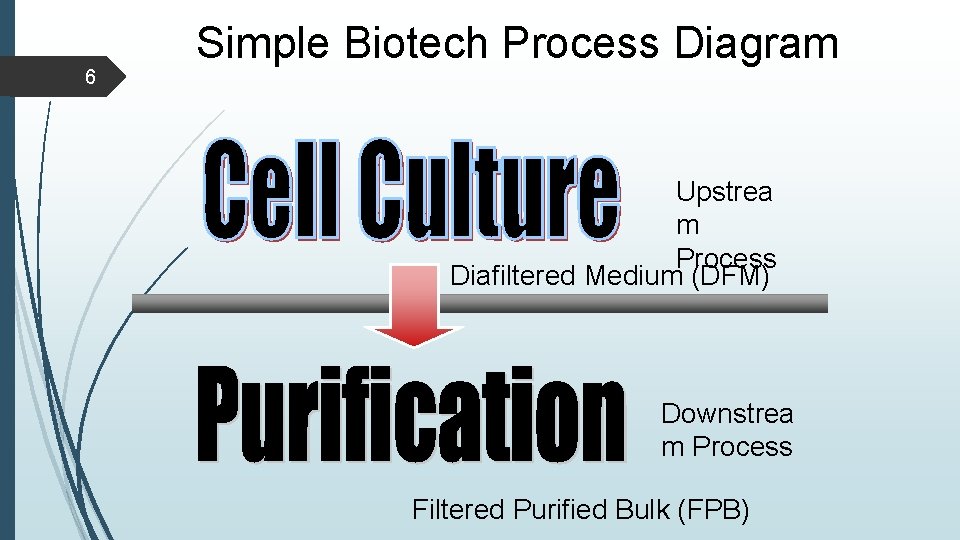
6 Simple Biotech Process Diagram Upstrea m Process Diafiltered Medium (DFM) Downstrea m Process Filtered Purified Bulk (FPB)

Typical Biotech Process 7

General Monoclonal Antibody Mfg Process 8

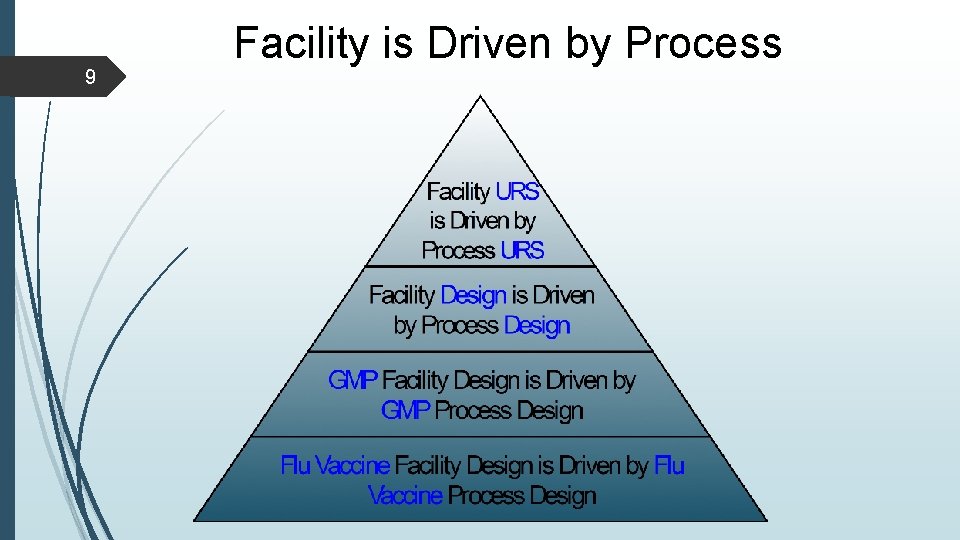
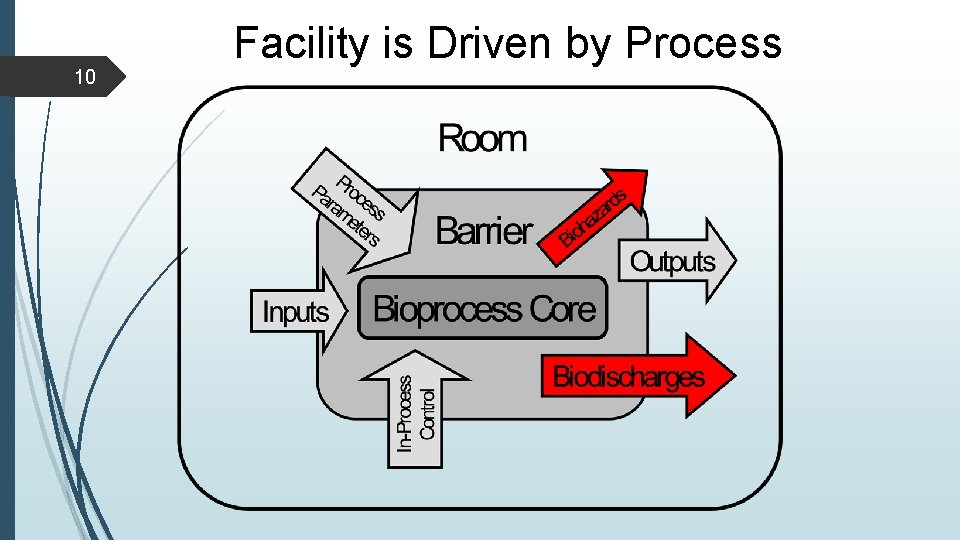
9 Facility is Driven by Process

10 Facility is Driven by Process

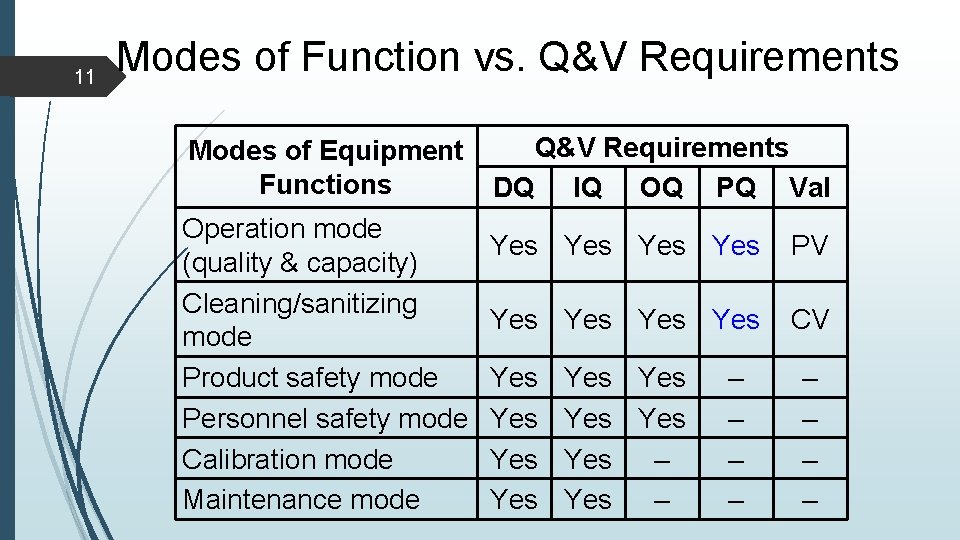
11 Modes of Function vs. Q&V Requirements Modes of Equipment Functions Operation mode (quality & capacity) Cleaning/sanitizing mode Product safety mode Personnel safety mode Calibration mode Maintenance mode Q&V Requirements DQ IQ OQ PQ Val Yes Yes PV Yes Yes CV Yes Yes Yes – – – – –

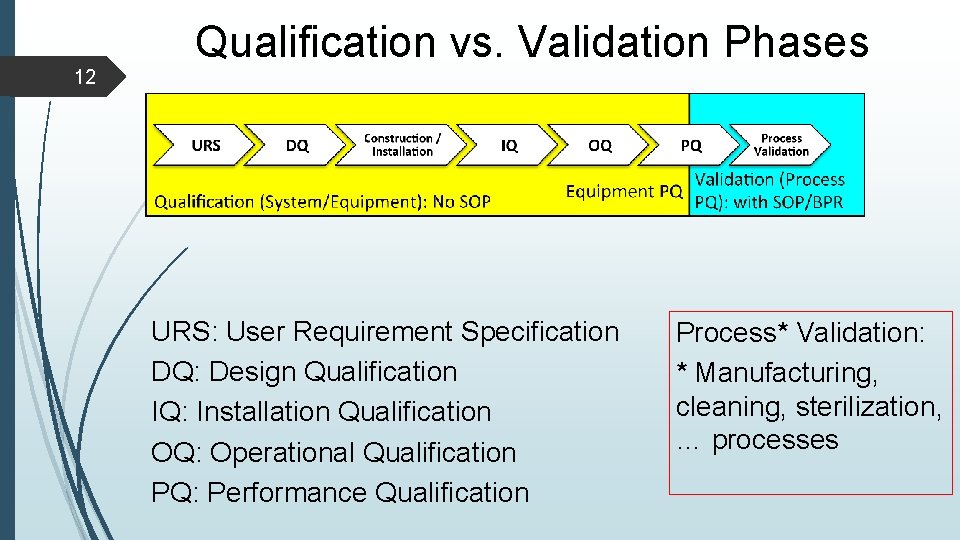
12 Qualification vs. Validation Phases URS: User Requirement Specification DQ: Design Qualification IQ: Installation Qualification OQ: Operational Qualification PQ: Performance Qualification Process* Validation: * Manufacturing, cleaning, sterilization, … processes

13 Regulations and Guidelines

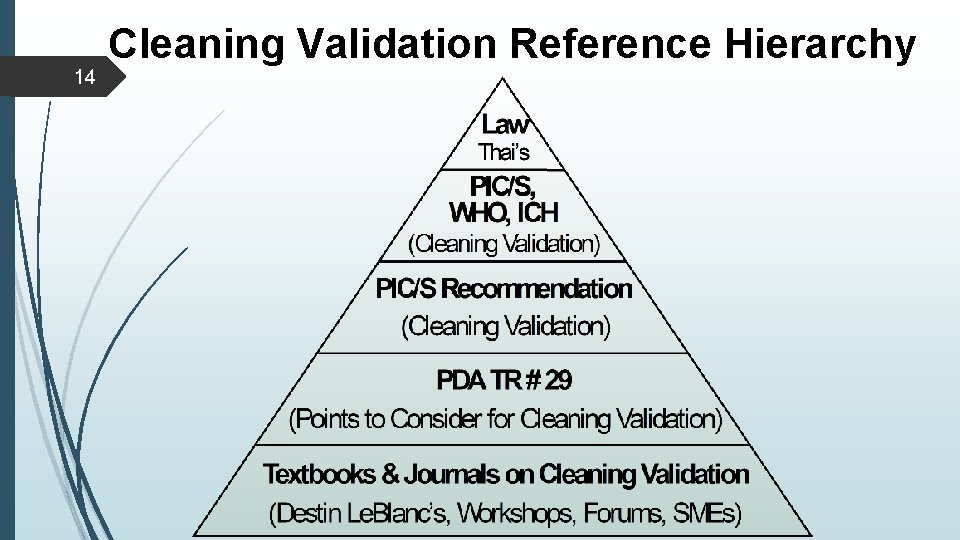
14 Cleaning Validation Reference Hierarchy

15

16 Reason, Definition and Concept

17 Reason for Cleaning Validation v Quality (with respect to Safety Attribute rather than Quality Attribute –i. e. hazardous-toconsumer or risky contaminants) v Regulatory Requirements i. e. GMP v Reliability of products i. e. index for the company’s effective Quality Management System (QMS)

18 CONTAMINANTS CHEMICAL RESIDUE DECOMPOSITION OF CHEMICAL CLEANING AGENT MICROBIAL (Bacteria, Mold, etc. ) AIRBORNE MATTER LUBRICANT …. , Etc.

19 Cleaning Validation Documented evidence that an approved cleaning procedure will provide equipment which is suitable (Consistently result) for proceeding of active pharmaceutical ingredients or pharmaceutical products. (acceptable predetermined level of cleanliness)



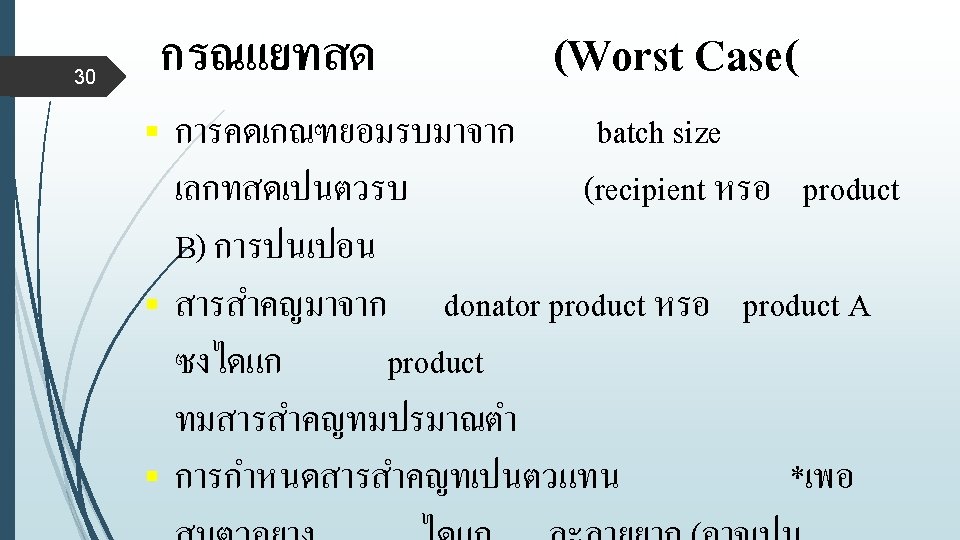
22 ระดบทปลอดภย FDA does not intend to set acceptance specifications or methods for determining whether a cleaning process is validated. The firm's rationale for the residue limits established should be logical based on the manufacturer's knowledge of the materials involved and be practical, achievable, and verifiable. (Validation of Cleaning Processes (7/93))

23

24 Regulation & Regulatory Requirements PIC/S Requirements

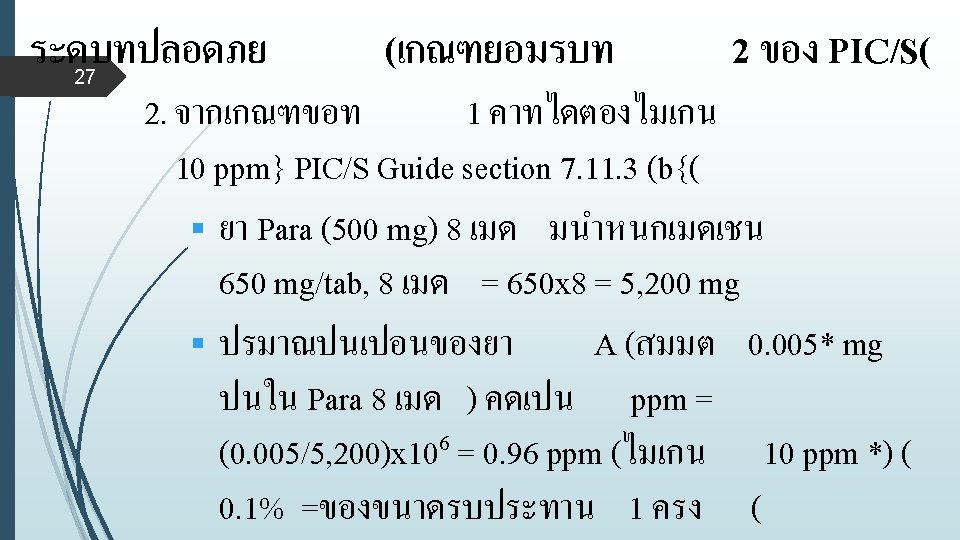
ขยายความเกณฑยอมรบท 1 ของ PIC/S (Concept) § คา 0. 1% หรอ 1/1000 คอ safety factor ซงใชกบผลตภณฑรบประทานท งหมด § ผลตภณฑกลมอนๆจะใช safety Factors Applied to factor- ดงน 1/100 Topical product 25 1/100 - 1/1, 000 Oral product 1/1, 000 - 1/10, 000 Injection , ophthalmic product 1/10, 000 - 1/100, 000 Research product










35 Where is cleaning validation required? (WHO) Not necessarily for non-critical cleaning, e. g. between batches of the same product (or different lots of the same intermediate in a bulk process), or of floors, walls, the outside of vessels, and following some intermediate steps. Considered important in multiproduct facilities -should be performed, e. g. for equipment, sanitization procedures and garment laundering. In addition to cleaning validation, if product is sold sterile, then sterility validation is required.

36 General Requirements (FDA) to have written procedures (SOP's) detailing the cleaning processes used for various pieces of equipment. to have written general procedures on how cleaning processes will be validated. the general validation procedures to address who is responsible for performing and approving the validation study, the acceptance criteria, and when revalidation will be required.

37 General Requirements (FDA) to prepare specific written validation protocols which should address such issues as sampling procedures, and analytical methods to be used including the sensitivity of those methods. to conduct the validation studies in accordance with the protocols and to document the results of studies. a final validation report which is approved by management and which states whether or not the cleaning process is valid. The data should support a conclusion that residues have been reduced to an "acceptable level. "

38 Activities on Cleanliness (ASTM) Clean How to validate clean lines and provide guidance Verify How to quantify residues on implants Design How to design implants with cleaning in mind

39 Clean Adequate removal of soils without introducing new residues Cleaning agents Migration of residues from one location to another Elution/extraction Damage to component or materials Mechanical, thermal, chemical Cost and process time Cross-contamination Multiple product lines

40 Cleaning

Verify 41 inspection consistency and for cleaning validation the test of any validation process is whether scientific data shows that the system consistently does as expected and produces a result that consistently meets predetermined specifications.

42 Cleaning Procedure


44


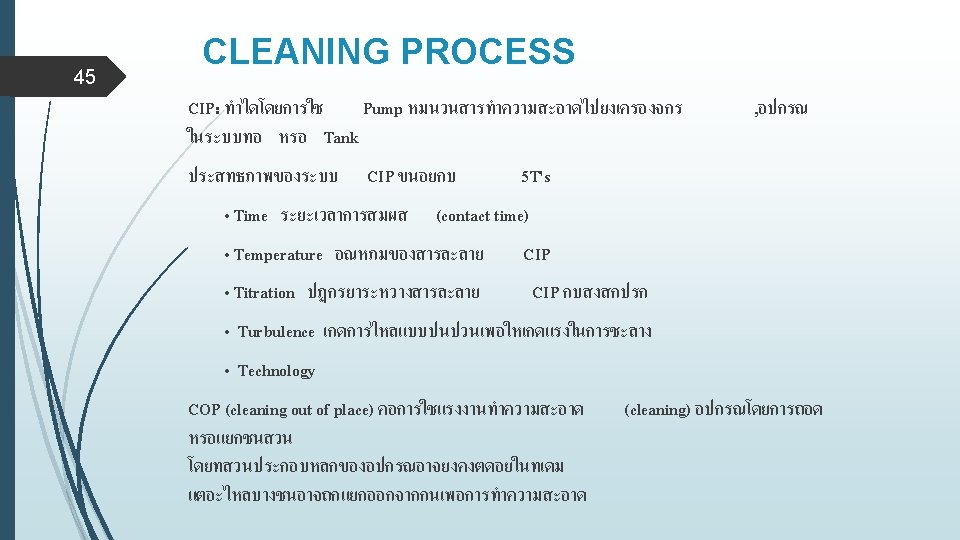
46 CLEANING PROCESS Clearly defined parameters & essential content temperature , time , pressure materials steps of action type & concentration of detergent rinsing ( amount , cycle , solvent ) clean equipment hold time and protection DEHT ( longest )(Dirty Equipment Hold Time)

47 CLEANING PROCESS

48 Cleaning Agent

49 CLEANING AGENTS Consideration effective cleaning Regulatory Compliance Know Component Safety Substrate compatibility Toxicity Stability and Shelf life Foaming Analyzability Microbial Control Disposal Manufacturing Quality/Standard Cost Effective

50 Microbiology

51 Microbiology Prevent microbial growth and remove contamination Documented evidence routine cleaning storage of equipment The period and conditions storage of unclean equipment before cleaning between cleaning and equipment reuse Equipment stored in a dry condition after cleaning (no stagnant water) Control of bioburden important

52 Microbiology Microbial requirements for sterile pharmaceutical drug products: Control of total bioburdento maintain the Sterility Assurance Level & elimination of endotoxinto prevent pyrogenicreactions. Endotoxinconsiderations: Endotoxinlimits forparenteralproducts are set using the maximum human dose for the individual products.

53

Validation Team 54 Validation Department Set appropriate Schedule plan Coordinate activities Prepare Documents from meeting resolution-set acceptance limit, set equipment sampling sites Lead & Drive to accomplish the Schedule plan. Production Department Prepare , Rectify …. . Cleaning Procedure Review & approve concerned documents -protocol, report, deviation, correction etc. Support related manufacturing & cleaning information Training/ Supervise / Inspect…. Cleaning process Record all variation / investigate the deviation

55 Validation Team Ø Engineering Department IQ, OQ , Calibration & preventive maintenance of E/Q Support related manufacturing & cleaning information. Drawing ( equipment train & diagram ) Calculate equipment surface area Communicates changes and evaluates equipment data Ø R & D //Quality Control Department Develop & Validate analysis method. -specificity, linearity, accuracy, precision, LOD, LOQ Calibration …. Measuring apparatus Perform Recovery studies Visual determination set up Review & approve concerned documents Training / supervise …. Sampling, Testing report with relevant data and obvious calculation.

56 Validation Team Quality Assurance Department Review & Approve all documents Inspect all activities to run correctly as determined in documents Change control
