Chemical Reactions Fireworks are a result of chemical










- Slides: 21

Chemical Reactions • Fireworks are a result of chemical reactions

Chemical Reactions • Chemical Reactions form new substances by breaking and making chemical bonds • Chemical reactions change the way the atoms are arranged

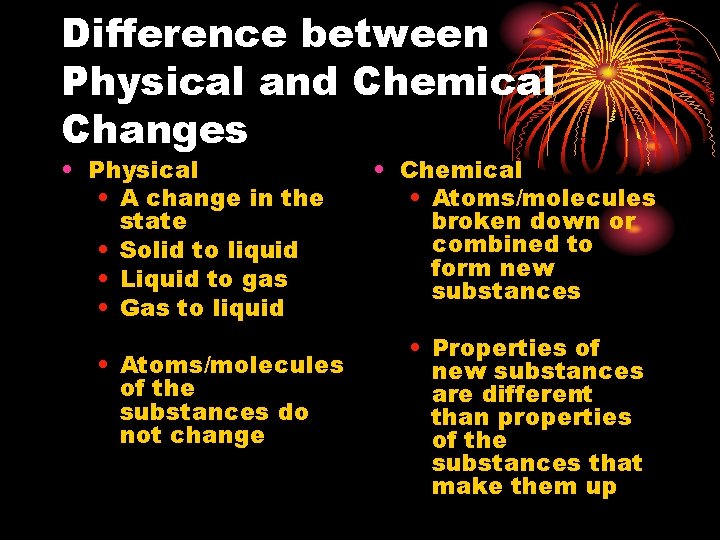
Difference between Physical and Chemical Changes • Physical • A change in the state • Solid to liquid • Liquid to gas • Gas to liquid • Atoms/molecules of the substances do not change • Chemical • Atoms/molecules broken down or combined to form new substances • Properties of new substances are different than properties of the substances that make them up

Reactants and Products • Reactants: present at the beginning of the reaction • Products: are the substances formed by the chemical reaction • Example burning natural gas • CH 4 + 2 O 2 > CO 2 +2 H 2 O • Reactants > Products

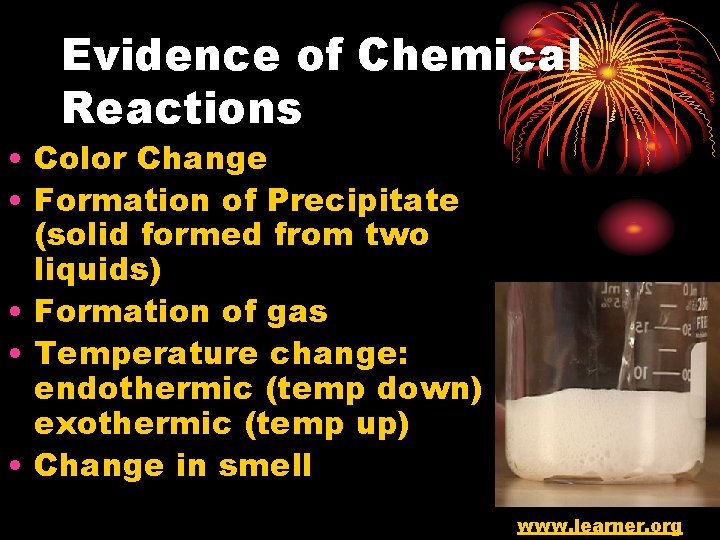
Evidence of Chemical Reactions • Color Change • Formation of Precipitate (solid formed from two liquids) • Formation of gas • Temperature change: endothermic (temp down) exothermic (temp up) • Change in smell www. learner. org

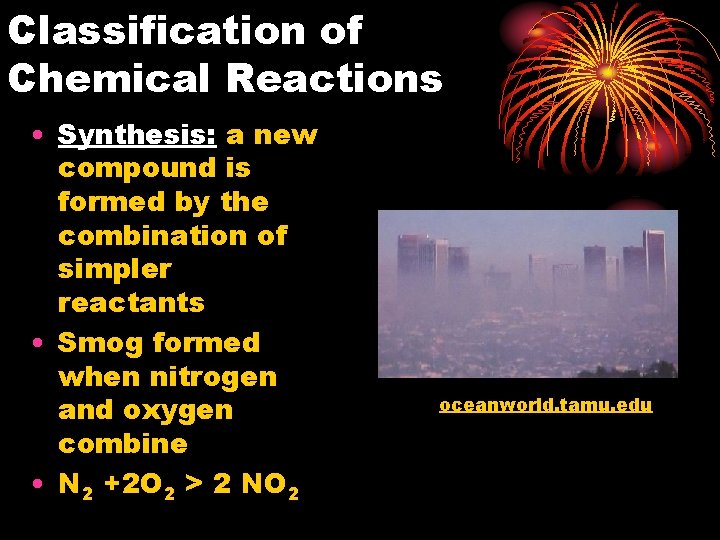
Classification of Chemical Reactions • Synthesis: a new compound is formed by the combination of simpler reactants • Smog formed when nitrogen and oxygen combine • N 2 +2 O 2 > 2 NO 2 oceanworld. tamu. edu

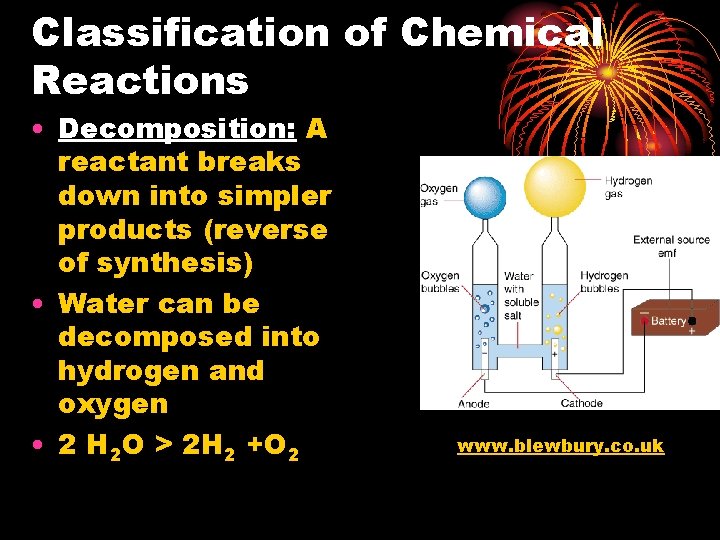
Classification of Chemical Reactions • Decomposition: A reactant breaks down into simpler products (reverse of synthesis) • Water can be decomposed into hydrogen and oxygen • 2 H 2 O > 2 H 2 +O 2 www. blewbury. co. uk

Classification of Chemical Reactions • Combustion: One reactant is always oxygen and another reactant often contains carbon and hydrogen • Burning of methane • CH 4 + 2 O 2 > CO 2 +2 H 2 O earthguide. ucsd. edu

The Rates of Chemical Reactions can vary • Concentration: measures the number of particles present in a certain volume. • A high concentration of reactants means there is a large number of particles that can collide and react • Turning the valve on a gas stove increases the methane molecules that can combine with oxygen and results in a bigger flame and faster combustion reaction

The Rates of Chemical Reactions can vary • Surface Area: the exposed surface of a substance. • To increase reaction break a large piece of the material so that there is more surface area of the material • Increase surface area=Increase reaction rate

Temperature • Rate of reaction can be increased by an increase in temperature which increases how fast the particles are moving and increases the number of collisions • Remember the cold and hot water distribution of food coloring

Catalyst • Catalyst: is a substance that increases the rate of chemical reaction but is not consumed in the reaction • Enzymes are catalysts that are used by living things to cause a chemical reaction. Resulting in a new product being made and the catalyst not being changed.

The masses of reactants and products are equal • Law of conservation of mass: states that in a chemical reaction atoms are neither created nor destroyed. (Antoine Lavoisier) • All atoms present in the reactants are also present in the products www. iuav. it

Chemical Equations • Reactants > Products • Balancing Chemical Equations • Use conservation of mass to balance: atoms can not be created or destroyed. • Reactants = products mooni. fccj. org

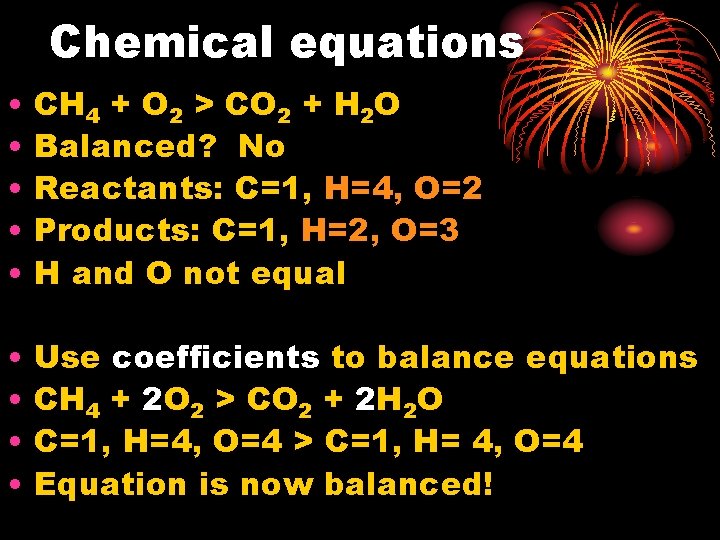
Chemical equations • • • CH 4 + O 2 > CO 2 + H 2 O Balanced? No Reactants: C=1, H=4, O=2 Products: C=1, H=2, O=3 H and O not equal • • Use coefficients to balance equations CH 4 + 2 O 2 > CO 2 + 2 H 2 O C=1, H=4, O=4 > C=1, H= 4, O=4 Equation is now balanced!

Chemical Reactions Involve Energy Changes • It takes energy to break chemical bonds. This energy is called bond energy. students. ed. uiuc. edu

Exothermic Reactions • Exothermic reaction: energy is released and temp goes up…more energy is released when products are formed. • Space shuttle on take off: white clouds of water vapor are formed www. sanchezcircuit. com

Exothermic Reactions www. learner. org • Exothermic reactions release energy! • All common combustion reactions are exothermic • Fireflies release energy in the form of light as an exothermic reaction

Endothermic reactions • Endothermic reactions: produce a decrease in temperature because the bond energies of the reactants are greater than the bond energies of the products • All endothermic reactions absorb energy! • Alka Seltzer and water…temperature goes down.

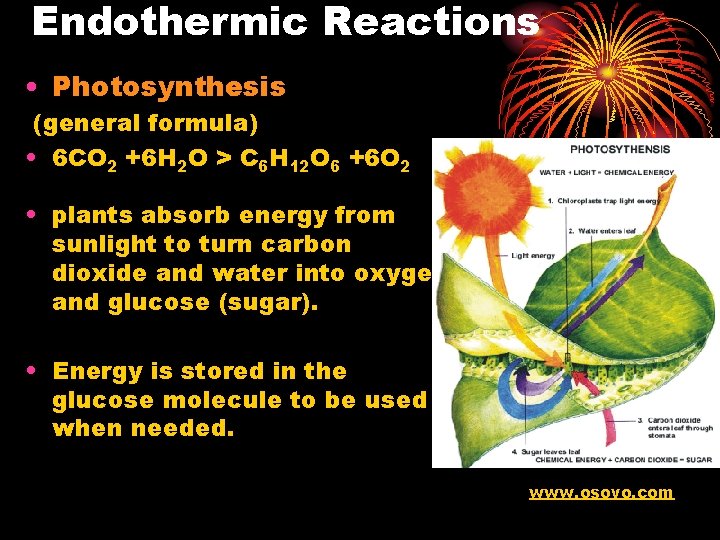
Endothermic Reactions • Photosynthesis (general formula) • 6 CO 2 +6 H 2 O > C 6 H 12 O 6 +6 O 2 • plants absorb energy from sunlight to turn carbon dioxide and water into oxygen and glucose (sugar). • Energy is stored in the glucose molecule to be used when needed. www. osovo. com

Life and Industry depend on Chemical Reactions • Living things require chemical reactions • Respiration: living things get energy from glucose by respiration. (combustion of glucose) • Photosynthesis • 6 CO 2 +6 H 2 O + energy> C 6 H 12 O 6 +6 O 2 • Respiration • C 6 H 12 O 6 +6 O 2 > 6 CO 2 +6 H 2 O + energy • Respiration is the opposite of photosynthesis!
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Antigentest åre
Antigentest åre Types of reactions
Types of reactions Is getting a haircut a physical change
Is getting a haircut a physical change Fireworks chemical equation
Fireworks chemical equation Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal How to write half reactions
How to write half reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers What does adobe fireworks do
What does adobe fireworks do Ken throws the discus at a school meet
Ken throws the discus at a school meet A ten inch fireworks shell is fired
A ten inch fireworks shell is fired Volcanoes nature's incredible fireworks
Volcanoes nature's incredible fireworks Projectile motion fireworks
Projectile motion fireworks Definition of adobe dreamweaver
Definition of adobe dreamweaver Fireworks forum
Fireworks forum Firework poems
Firework poems Equilibrium of chemical reactions
Equilibrium of chemical reactions What is released or absorbed whenever chemical
What is released or absorbed whenever chemical 5 general types of chemical reactions
5 general types of chemical reactions Grade 11 chemistry unit 4
Grade 11 chemistry unit 4