Chemical Reactions and Enzymes Living things undergo thousands


- Slides: 12

Chemical Reactions and Enzymes Living things undergo thousands of chemical reactions as part of the life process Many are very complex involving multistep sequences called biochemical pathways

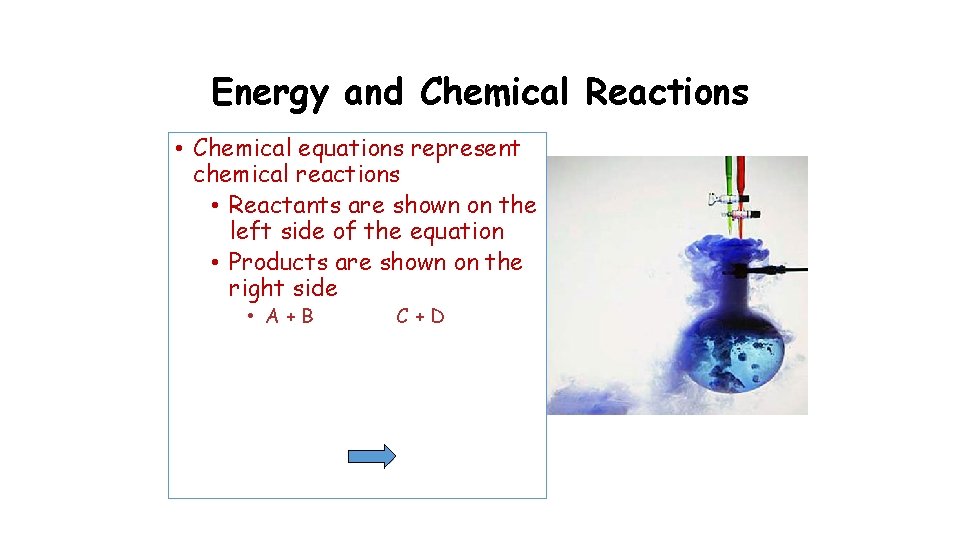
Energy and Chemical Reactions • Chemical equations represent chemical reactions • Reactants are shown on the left side of the equation • Products are shown on the right side • A+B C+D

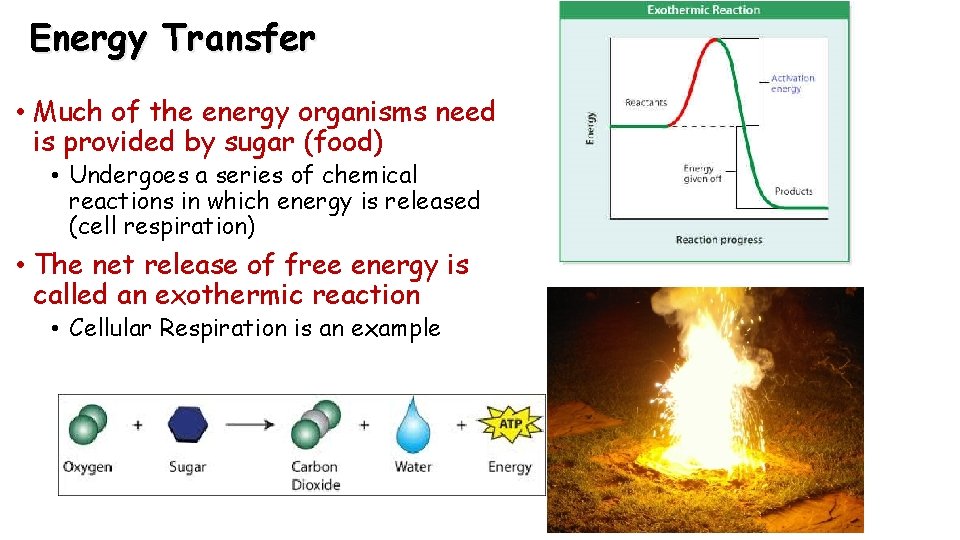
Energy Transfer • Much of the energy organisms need is provided by sugar (food) • Undergoes a series of chemical reactions in which energy is released (cell respiration) • The net release of free energy is called an exothermic reaction • Cellular Respiration is an example

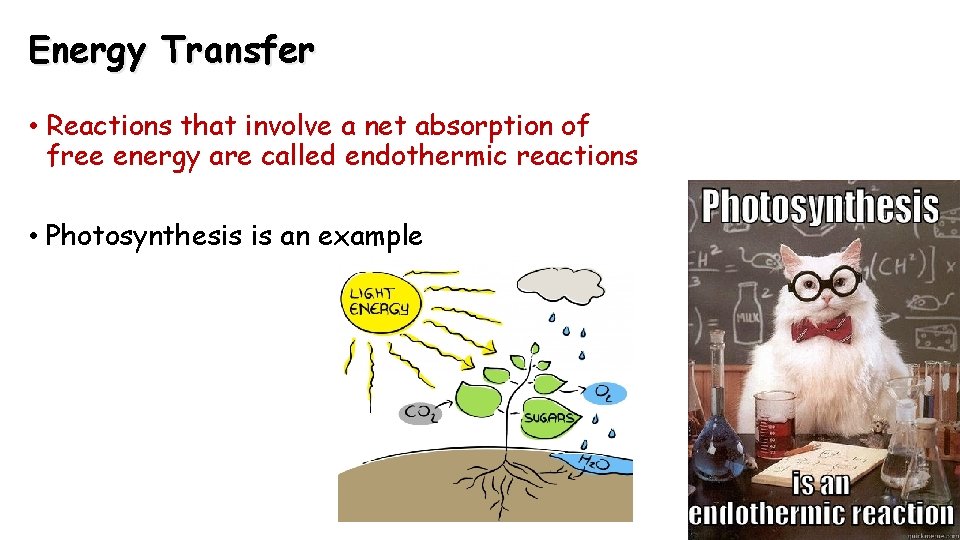
Energy Transfer • Reactions that involve a net absorption of free energy are called endothermic reactions • Photosynthesis is an example

Energy Transfer • Most chemical reactions require energy to begin • The amount of energy needed to start the reaction is called activation energy

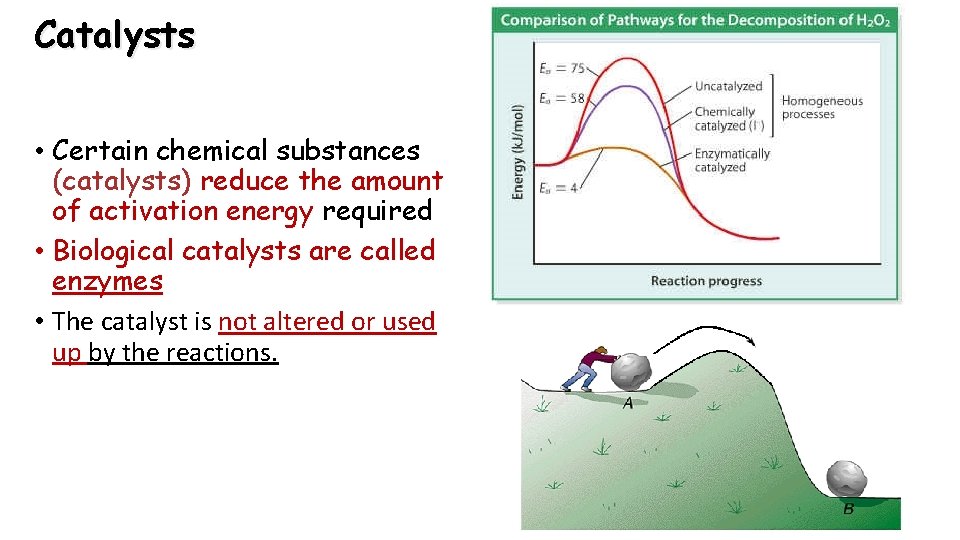
Catalysts • Certain chemical substances (catalysts) reduce the amount of activation energy required • Biological catalysts are called enzymes • The catalyst is not altered or used up by the reactions.

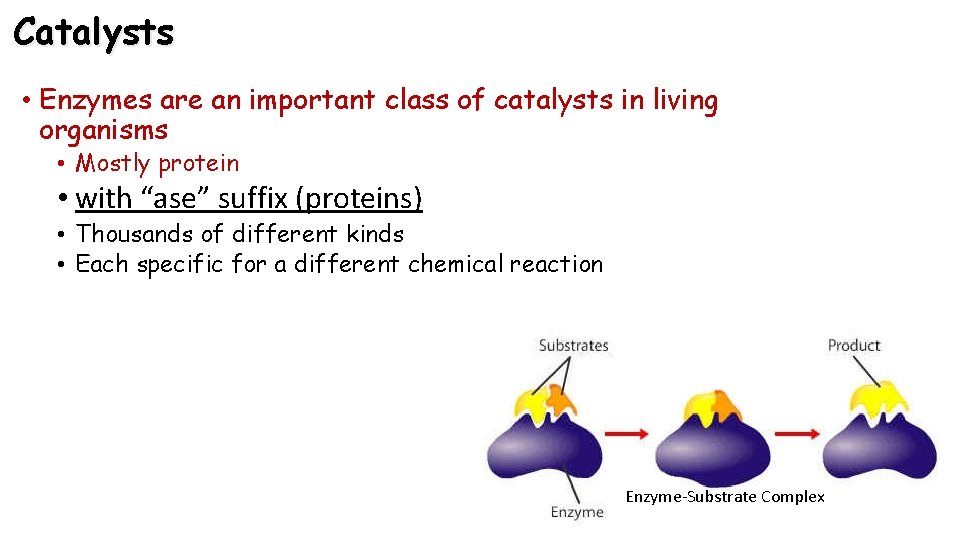
Catalysts • Enzymes are an important class of catalysts in living organisms • Mostly protein • with “ase” suffix (proteins) • Thousands of different kinds • Each specific for a different chemical reaction Enzyme-Substrate Complex

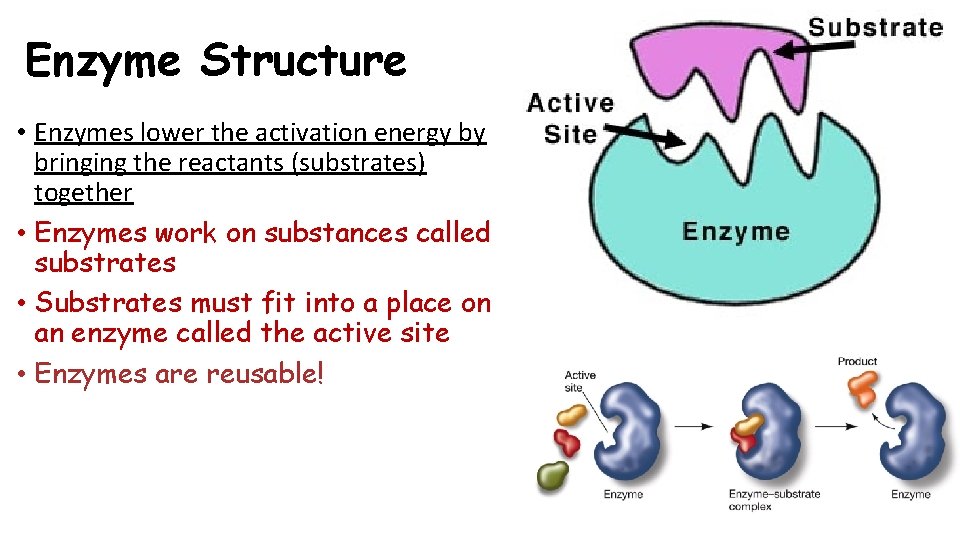
Enzyme Structure • Enzymes lower the activation energy by bringing the reactants (substrates) together • Enzymes work on substances called substrates • Substrates must fit into a place on an enzyme called the active site • Enzymes are reusable!

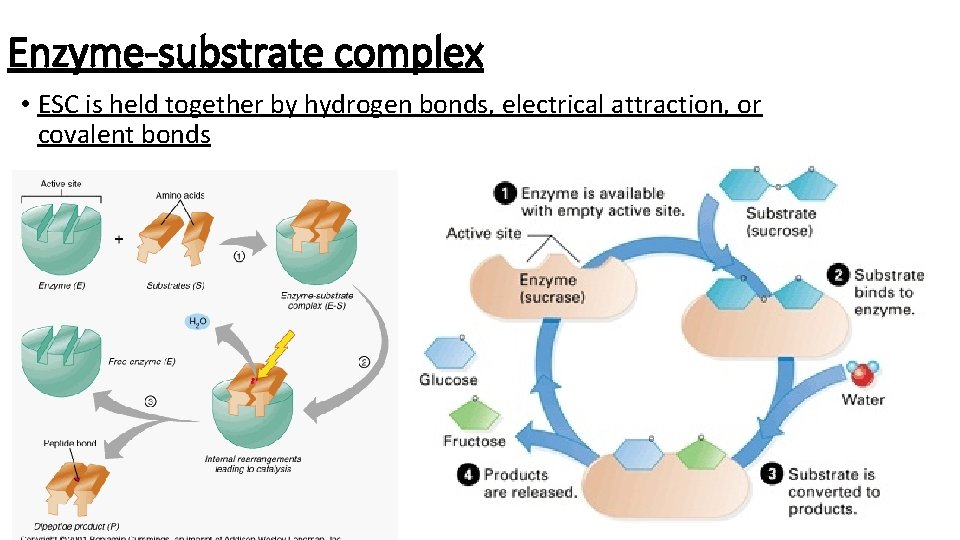
Enzyme-substrate complex • ESC is held together by hydrogen bonds, electrical attraction, or covalent bonds

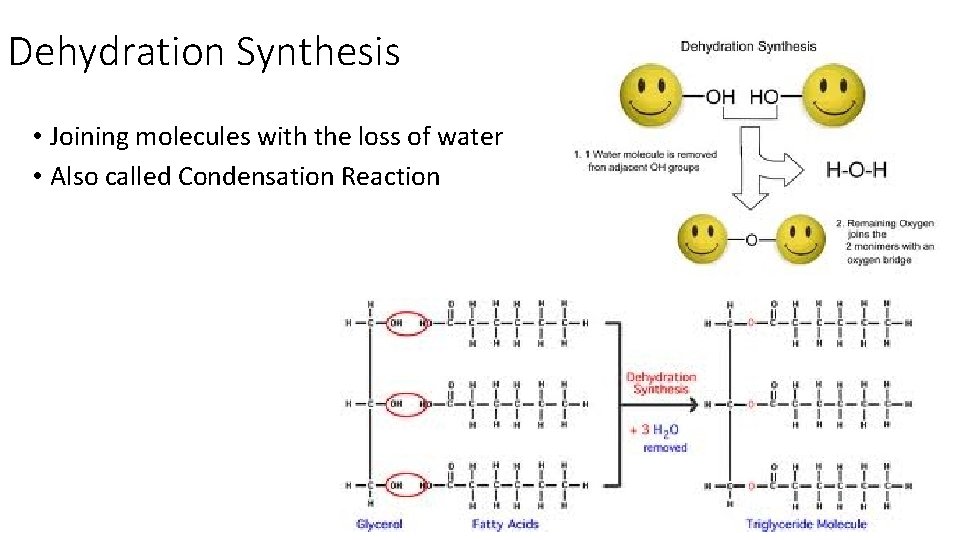
Dehydration Synthesis • Joining molecules with the loss of water • Also called Condensation Reaction

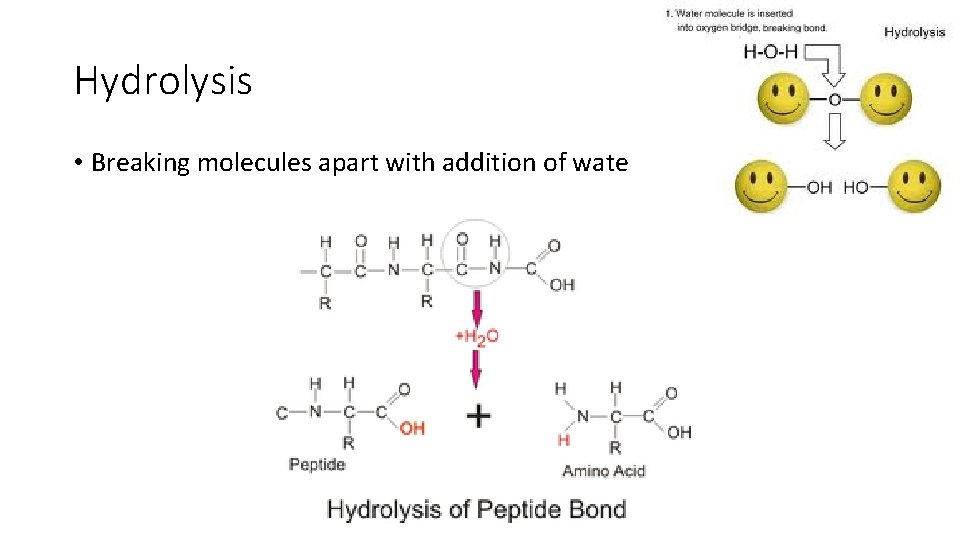
Hydrolysis • Breaking molecules apart with addition of water

PROTEINS AS ENZYMES • Create your own enzyme that increases the speed of a particular metabolic reaction. This can be either dehydration synthesis or hydrolysis. • Draw your enzyme, its substrate and active site. • Label each part. • Describe what your enzyme does using complete sentences.
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Enzymes affect the reactions in living cells by
Enzymes affect the reactions in living cells by Www.biology-roots.com
Www.biology-roots.com How are enzymes important to living things
How are enzymes important to living things Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Blue things
Blue things Smallest living unit
Smallest living unit Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal