Chem E 260 Polytropic Isentropic Processes Dr William



- Slides: 13

Chem. E 260 Polytropic & Isentropic Processes Dr. William Baratuci Senior Lecturer Chemical Engineering Department University of Washington TCD 7: E CB 6: 3 - 5 May 9, 2005

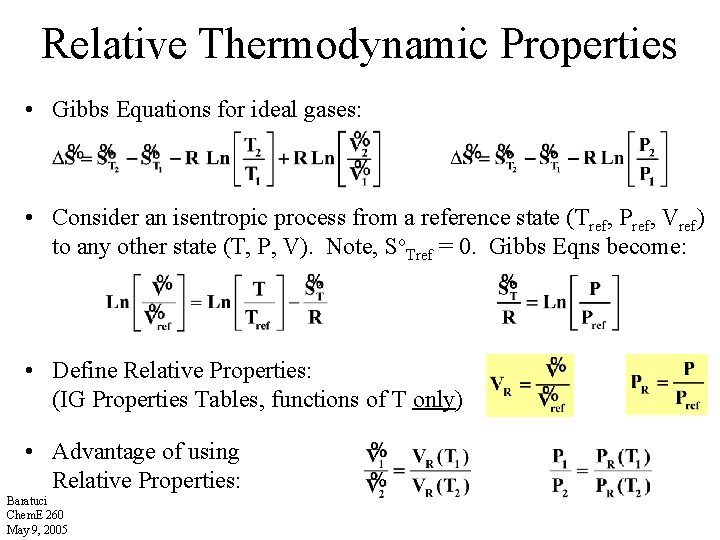
Relative Thermodynamic Properties • Gibbs Equations for ideal gases: • Consider an isentropic process from a reference state (Tref, Pref, Vref) to any other state (T, P, V). Note, So. Tref = 0. Gibbs Eqns become: • Define Relative Properties: (IG Properties Tables, functions of T only) • Advantage of using Relative Properties: Baratuci Chem. E 260 May 9, 2005

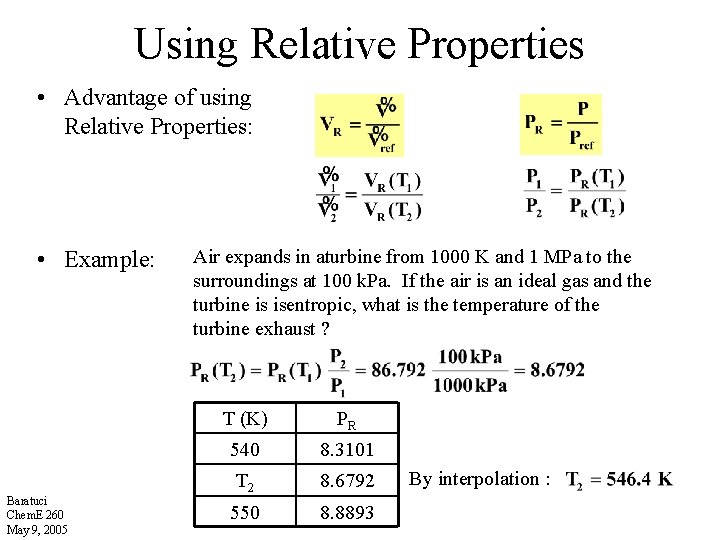
Using Relative Properties • Advantage of using Relative Properties: • Example: Baratuci Chem. E 260 May 9, 2005 Air expands in aturbine from 1000 K and 1 MPa to the surroundings at 100 k. Pa. If the air is an ideal gas and the turbine is isentropic, what is the temperature of the turbine exhaust ? T (K) PR 540 8. 3101 T 2 8. 6792 550 8. 8893 By interpolation :

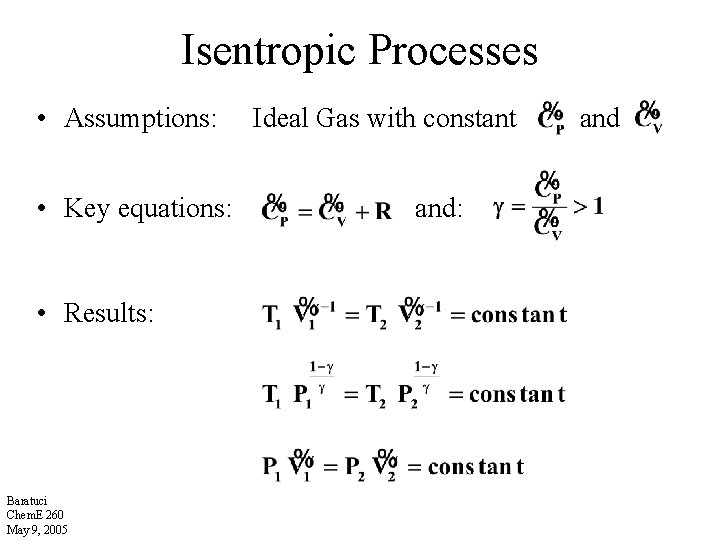
Isentropic Processes • Assumptions: • Key equations: • Results: Baratuci Chem. E 260 May 9, 2005 Ideal Gas with constant and: and

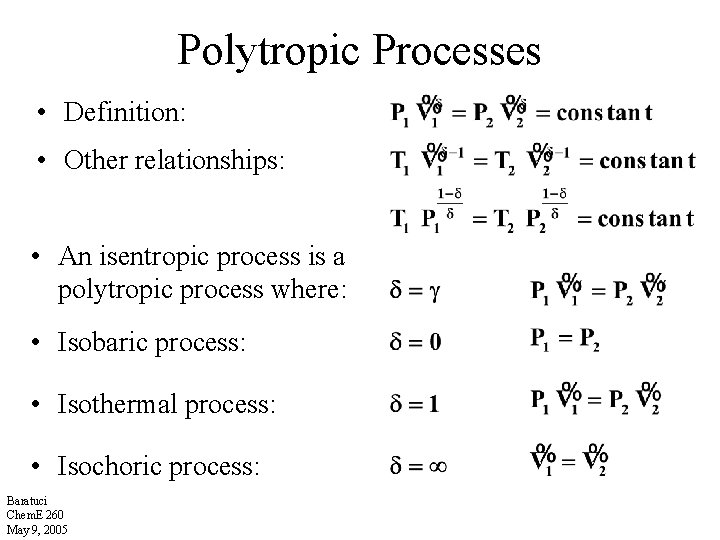
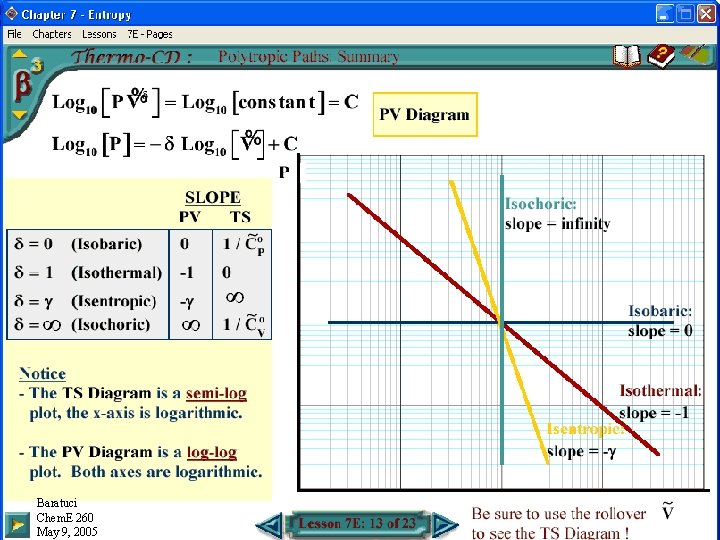
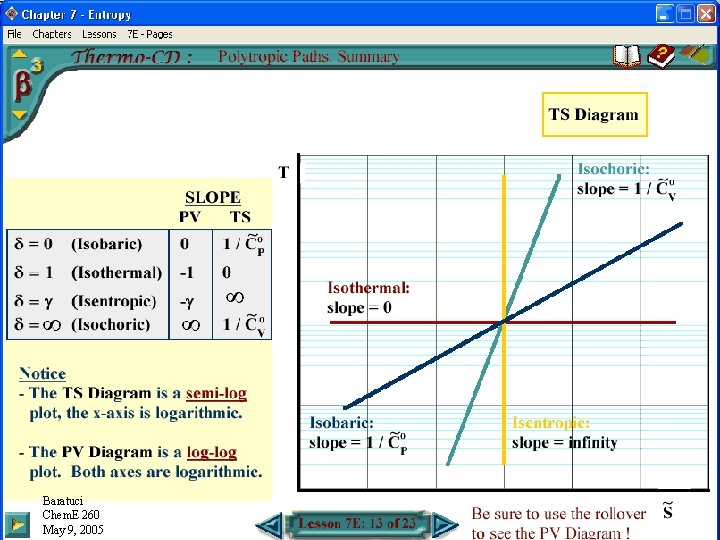
Polytropic Processes • Definition: • Other relationships: • An isentropic process is a polytropic process where: • Isobaric process: • Isothermal process: • Isochoric process: Baratuci Chem. E 260 May 9, 2005

PV Diagram for Polytropic Processes Baratuci Chem. E 260 May 9, 2005

TS Diagram for Polytropic Processes Baratuci Chem. E 260 November May 16, 9, 2004 2005

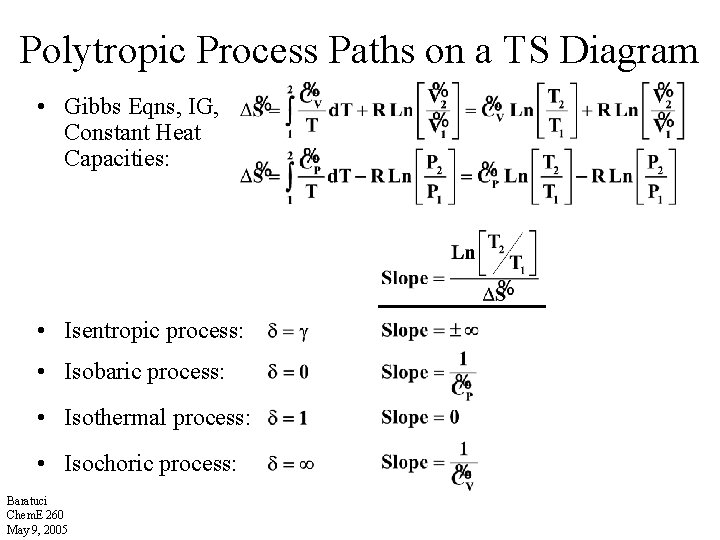
Polytropic Process Paths on a TS Diagram • Gibbs Eqns, IG, Constant Heat Capacities: • Isentropic process: • Isobaric process: • Isothermal process: • Isochoric process: Baratuci Chem. E 260 May 9, 2005

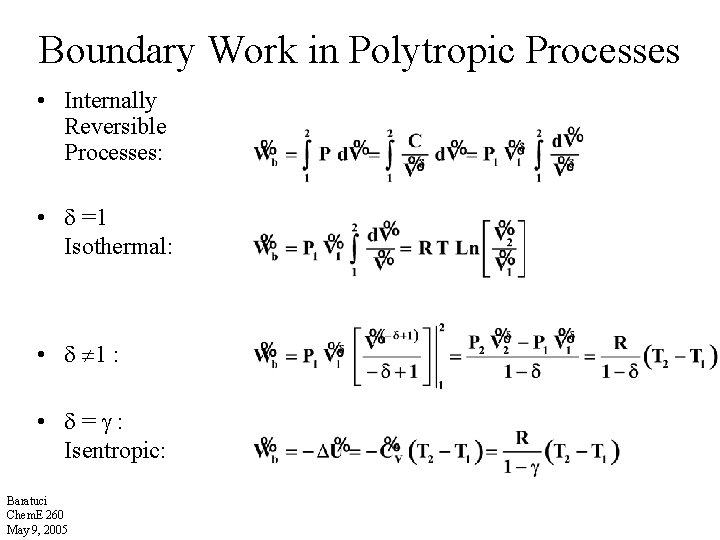
Boundary Work in Polytropic Processes • Internally Reversible Processes: • =1 Isothermal: • 1 : • = : Isentropic: Baratuci Chem. E 260 May 9, 2005

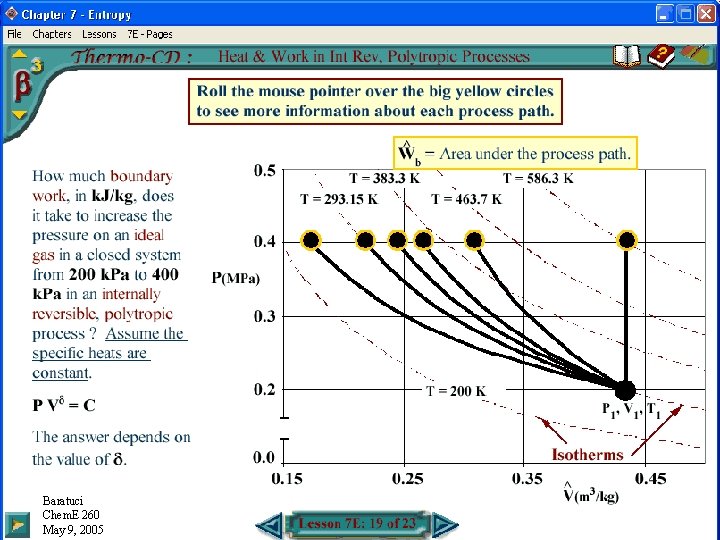
Wb: PV Diagram Baratuci Chem. E 260 November May 16, 9, 2004 2005

Next Class … • Entropy Balances – Closed Systems: No Mass Crosses the System Boundary – Open Systems: Mass Crosses the System Boundary • Entropy Generation and Suniv • The Mechanical Energy Balance Equation – The Bernoulli Equation • PV Diagrams Revisited – Shaft Work on a PV Diagram ! Baratuci Chem. E 260 May 9, 2005

Example #1 • Saturated ammonia vapor enters an adiabatic compressor at -10 o. C and leaves at a pressure of 1. 0 MPa. Determine the work requirement per kg of ammonia for the compressor if the process is reversible.

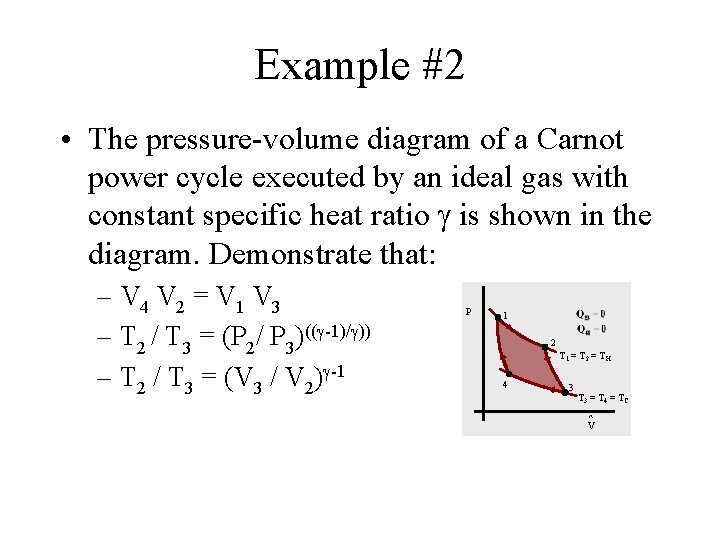
Example #2 • The pressure-volume diagram of a Carnot power cycle executed by an ideal gas with constant specific heat ratio is shown in the diagram. Demonstrate that: – V 4 V 2 = V 1 V 3 – T 2 / T 3 = (P 2/ P 3)(( -1)/ )) – T 2 / T 3 = (V 3 / V 2) -1 P 1 2 T 1 = T 2 = TH 4 3 T 3 = T 4 = TC ^ V