Department of Mechanical Engineering ME 322 Mechanical Engineering

- Slides: 9

Department of Mechanical Engineering ME 322 – Mechanical Engineering Thermodynamics Lecture 22 Second Law Analysis of Open Systems Isentropic Device Efficiency

Isentropic Efficiency of Devices • Performance index of a device • Comparison of device performance relative to an isentropic process – The isentropic process is reversible + adiabatic – The isentropic process represents the limiting performance of the device • Not achievable in the real-world • A great benchmark for design and analysis • Limiting value = 100%! • No general expression – Device specific 2

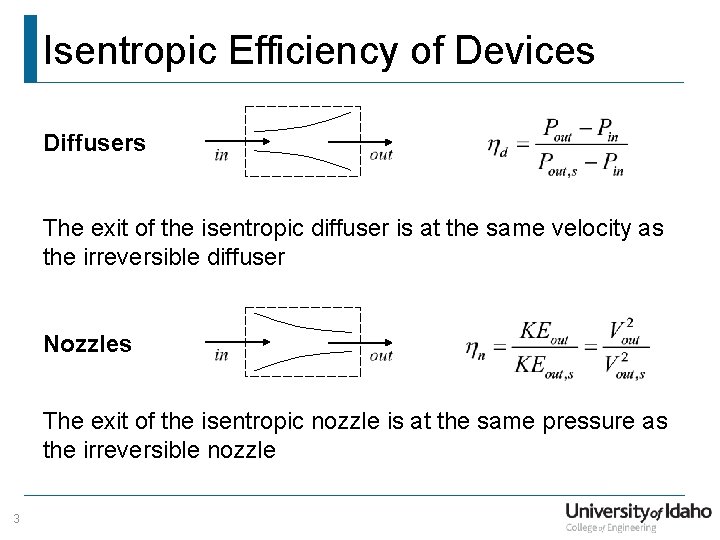
Isentropic Efficiency of Devices Diffusers The exit of the isentropic diffuser is at the same velocity as the irreversible diffuser Nozzles The exit of the isentropic nozzle is at the same pressure as the irreversible nozzle 3

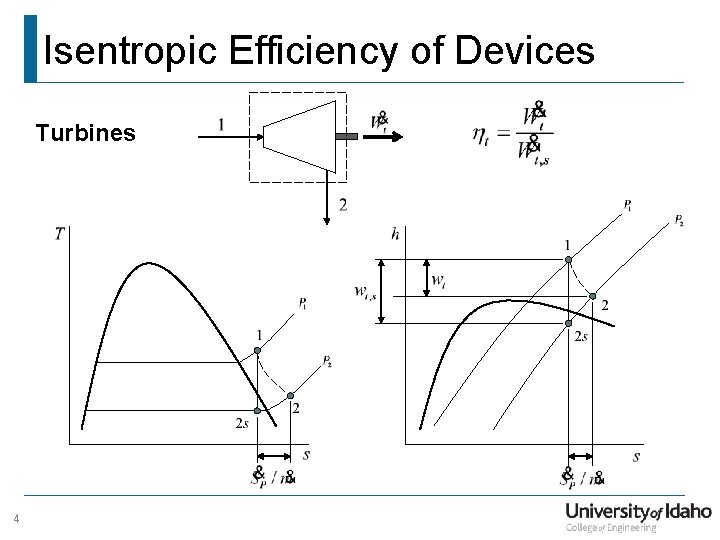
Isentropic Efficiency of Devices Turbines 4

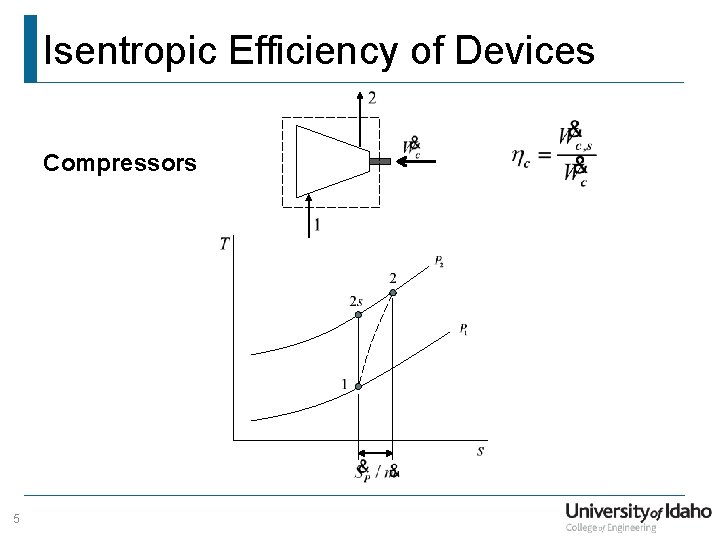
Isentropic Efficiency of Devices Compressors 5

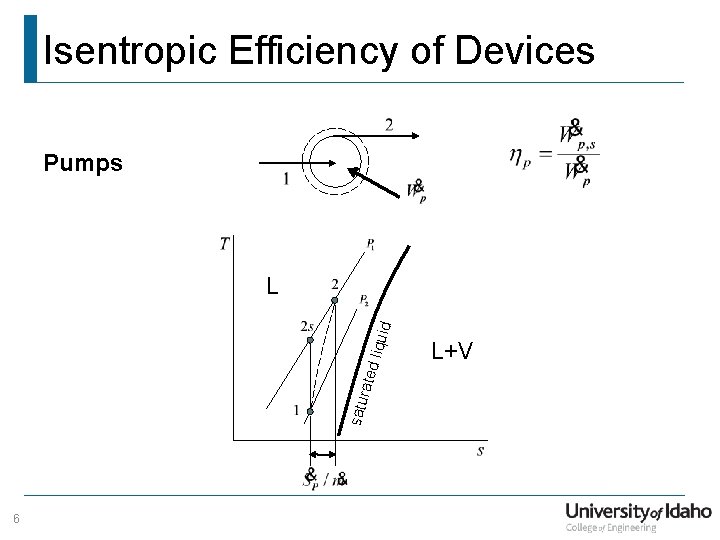
Isentropic Efficiency of Devices Pumps satu rate d liq uid L 6 L+V

Isentropic Efficiency of Devices What about throttling processes and heat exchangers? Throttling Process There is no ‘reversible’ analog to a throttling process. Specifying an ‘isentropic’ throttling results in an enthalpy change across the device. And a delta_h implies work is involved. **As a result, we do not define an isentropic efficiency for such a device. Heat Exchangers Heat exchangers operate by virtue of a temperature difference between the fluids. By definition, this is irreversible heat transfer. For heat transfer to be reversible there must be no temperature difference driving the heat transfer. **As a result, we do not define an isentropic efficiency for such a device. 7

Example – air turbine w/Air Tables Air expands in an air turbine from a pressure of 450 k. Pa and a temperature of 550 K to an exhaust pressure of 150 k. Pa. Assume the process is reversible and adiabatic, with negligible changes in kinetic and potential energy. a) Sketch the system & boundary and classify the system. b) Show initial state and final state isobars on both Pv and Ts diagrams. Sketch this process on both diagrams. Visualize and forecast sign of work as well as heat transfer. c) Use the air tables to find initial and final state conditions. d) Write the conservation of energy for this process. e) Write the entropy balance for this process. f) What is the specific work of air flowing through the turbine? g) What is the specific entropy production for this turbine? 8

Example – steam turbine A steam turbine receives steam at a pressure of 1 MPa, 300 C. The steam leaves the turbine at a pressure of 15 k. Pa. The work output of the turbine is measured and found to be 600 k. J/kg of steam flowing through the turbine. a) Sketch the system & boundary and classify the system. b) Show initial state and final state isobars on a Ts diagram. Sketch the ideal and real process on the Ts diagram. c) Use the steam tables to find real and ideal final state conditions. d) What is the quality at the turbine outlet (both real and ideal)? e) Write the conservation of energy for this process. f) What is the specific work for an ideal turbine with these conditions? g) What is the isentropic efficiency of the turbine? h) What is the specific entropy production for the real turbine? 9